Cell Sheet Tissue Engineering, by Thomas Fernberg and Julia Tomaszewski
Introduction
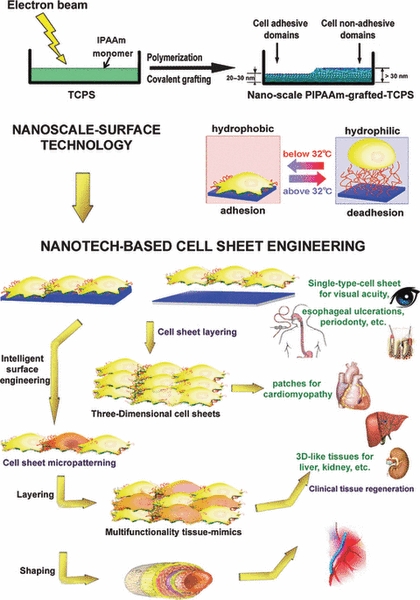
Cell sheet tissue engineering is a scaffold-free approach to regenerating damaged tissue cells. This is a fairly new technology, originating in 1990 [1]. Prior to cell sheet tissue engineering, scaffolds were the primary successful cell-based therapy to restore damaged tissue.
A variety of cell types may be used in order to regenerate tissue depending on which type of tissue is damaged. Cell sheets are engineered by culturing the desired cells on a thermo-responsive polymer surface (PIPAAm) [2]. This is necessary for the detachment of the cells from the culture surface. Cells proliferate on this polymer surface, creating cell-cell junctions from multi-protein complexes, which hold the sheet together. The sheet maintains its extracellular matrix (ECM) when detached from the culture surface which is beneficial for cell adhesion to the host. Cell sheets can range between 5-600 μm in thickness [3] and are therefore layered by placing the sheets on top of one another in order to produce a more cell dense, three dimensional structure [2]. Once the cell sheets have been cultured, they are implanted into a patient and regenerate the damaged tissue.
Motivation
Cell regeneration therapy is an essential, developing technology for the increasing demand of tissue reconstruction. Organ transplants are the ideal treatment for many patients with tissue damage, but the demand of organs surpasses available organs for transplantation.
There are several types of cell-based regenerative therapies currently being applied, including injection of isolated cells, scaffold engineering, and cell sheet tissue engineering. Isolated cell injection has a low cell survival rate once transplanted into a host, which takes away from their therapeutic benefits [2]. Therefore, other methods such as scaffolds and cell sheets have been engineered to increase survival rates of cells in the tissue and improve the tissue function of the patients.
Scaffolds are a successful means to regenerate tissue, but may have damaging affects in sensitive organs. The main drawback to scaffolds is the inflammatory response once the scaffold degrades, which hinders the cell viability and therapeutic effects [4][5]. Also, scaffolds may inhibit passive diffusion of nutrients and can accumulate wastes within them [4].
Temperature-Responsive Polymer Surface
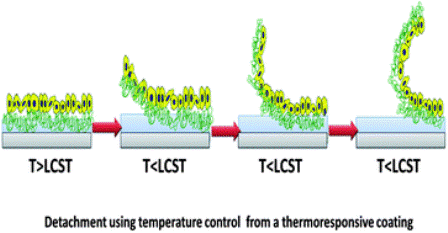
Cell sheets are designed on a surface that supports cell growth and have cell adhesion/dehesion characteristics. Originally, cell sheets were removed from culture surfaces by proteolytic enzymes, such as trypsin, pronase, or collagenase. This approach hindered functionality of the cell sheets because the enzymes would disrupt the membrane-associated proteins, distressing the cell-cell junctions that hold the sheet together [1].
This led to the design of a thermo-responsive polymer surface called Poly(N-isopropylacrylamide) (PIPAAm) [1]. PIPAAm is a polymer that is grafted to cell culture surfaces and is be controlled by varying temperatures. Its reversible temperature responsive characteristics allow the cells to preserve their functionality.
Temperature-responsive culture dishes are prepared several ways, but the most common is by the addition of a monomer, N-isoproplyacrylamide (IPAAm), to a culture dish and applying electron beam (EB) irradiation [2][6]. This process covalently immobilizes PIPAAm to the culture surface [1]. PIPAAm surfaces are typically 20-30 nm thick. A PIPAAm thickness greater than 30 nm inhibits cell adhesion and response from temperature variations, therefore the polymer thickness is usually maintained at 20 nm.
PIPAAm’s hydrophobic and hydrophilic qualities when exposed to different temperatures are what make this polymer a desirable material to graft a culture surface. Temperatures above PIPAAm’s lower critical solution temperature (LCST), 32°C, are hydrophobic and ideal for cell adhesion, and temperatures below its LCST are hydrophilic [1]. Therefore, cell sheets are generally cultured at 37°C because cells are able to facilitate attachment and proliferation easily [2]. Once cells have proliferated, the temperature is lowered to 20°C. In hydrophilic conditions, a layer of water forms between the cell sheet and the polymer surface [7], inducing competition for binding between the water molecules and the cell sheet, allowing the cell sheet to disassociate from the polymer without damage [1]. This is beneficial because, unlike applying enzymes to the cells, the sheets retain their biological activity, functionality, and their ECM proteins.
Because engineered cell sheets are able to maintain their ECM, the sheets can be layered to create a three dimensional structure. Typically, cell sheets contain three layers but are able to support many layers depending on the desired thickness of the cell sheet. Also, the presence of ECM on the basal cell sheet surface allows for a simple transplantation of the cell sheets to a patients tissue [2].
Applications of Cell Sheets
Corneal Reconstruction
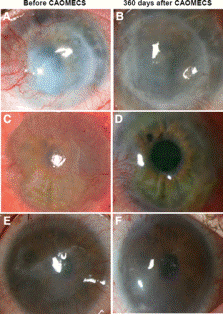
Limbal stem-cell deficiency (LSCD) due to trauma or disease of the cornea can cause corneal opacification or loss of vision [7]. LSCD is caused by limbal epithelial stem cell dysfunction or degradation and can be repaired with cell sheet technology [8]. The outer-most layer of the cornea, the epithelium, is most susceptible to damage. In patients with unilateral disease, epithelial cells from the autologous cornea can be used for cellular generation. Patients with bilateral disease are treated with epithelial cells from the oral mucosa. Oral mucosa epithelial cells are the most similar to those of corneal epithelial cells and can deliver a high level of optical transparency for the eye, although they are not as effective as corneal epithelial cells.
To transplant the cultured epithelial cell sheet to the damaged cornea, the sheet is directly placed on the stromal bed (middle layer of the cornea) [8]. The cell sheet diameter is typically about 20 mm [8] but can be altered if necessary.
In clinical trials, many patients experienced dry eye occurring from a tear production malfunction which was corrected with artificial tears [8]. Also, some patients experienced other issues such as inflammation and neovascularization. Results prove that this technology can produce long term benefits for patients with LCSD [7].
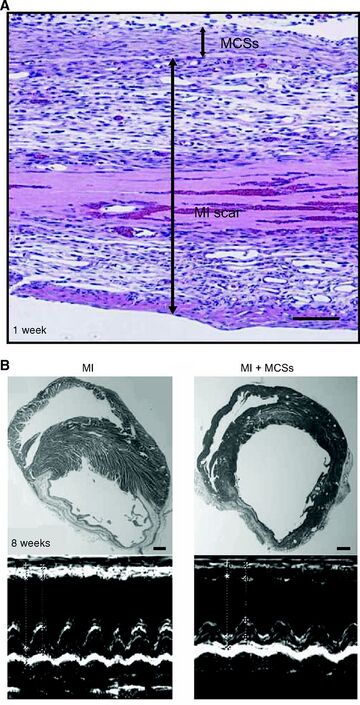
Cardiac Tissue Regeneration
Myocardial infarction (MI) is one of the leading causes of heart failure which a major cause of sickness and mortality in developed countries [9]. One percent of people worldwide over the age of 65 develop some type of heart ailment [10]. Cardiac transplantation is the leading treatment of heart failure, but the competition for organ donation is very high and only about 4,000 patients per year requiring this surgery receive a cardiac transplantation [10]. Other methods, such as cell transplantation via injection, may aggregate or kill the cells due to harm from the injection needle in over 85% of cells [9][10]. Scaffolds also may not deliver a suitable treatment of cell regeneration. This is because scaffolds are unable to regenerate dense myocardial tissue which is necessary for proper function of the cardiac muscle. Also, cardiomyocyte alignment is difficult to achieve via use of scaffolds [10]. Therefore, the application of cell sheets is a desirable alternative method of therapy.
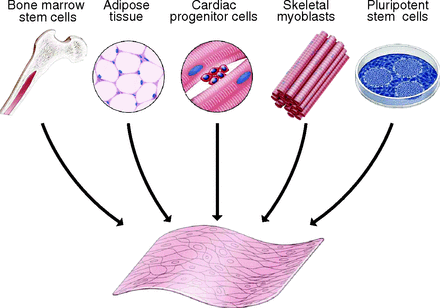
To regenerate cardiac tissue, an alternate source of cells must be utilized because the cultivation of cardiomyocytes is difficult. Skeletal myoblasts (SMs), bone marrow stem cells (BMSCs), adipose derived stem cells (ADSCs), and cardiac progenitor cells (CPCs) are potential cell sources to regenerate cardiac tissue [10].
Mesenchymal stem cells (MSCs) are the most promising cells for tissue regeneration because they can be found in various cell types, such as BMSCs and ADSCs. They also have the ability to differentiate to other types of cells, including cardiomyocytes (CMs), which are thought to be the most advantageous source of MSCs [10]. This is because cell sheets cultured from CMs have proved to aid in cardiac contraction, provide and ideal heart rhythm, and are able to connect with other CM cell junctions in the host [10].
Once CM cell sheets are layered and administered an electrical stimulant in vitro, they spontaneously pulse, mimicking actual CM cells in the body. When CM cell sheets are implanted into a host, they are able to mimic the activity of the host's own CM cells, assisting in the cardiac function [4]. This study has been conducted in rats and other animals with success, but problems still persist.
Future Directions
Creation of Organ-Like Structures
Cell sheets may also be used in the construction of organ-like structures. Research to replace the myocardial chamber in rats is being analyzed [7]. The objective of this research is for the cell sheet organ structure to operate as a healthy myocardial chamber would. This design requires the organ structure to spontaneously contract and pulse from stimulants in the host.
To fabricate this structure, six CM cell sheets are layered and wrapped around the aorta creating a cylindrical structure, and is then implanted into the host. Experimentation shows that these organ structures function similarly to those of undamaged myocardium [7]. This approach has been tested in rats but research is still in development.
References
[1] Yamada, N., Okano T., Sakai, H., Karikusa, F., et al. Thermoresponsive Polymeric Surfaces - Control of Attachment and Detachment of Cultured-Cells. Makromolekulare Chemie-Rapid Communications. 1990;11:571-576.
[2] Haraguchi, Yuji, Shimizu, Tatsuya, Yamato, Masayuki, et al. Scaffold-free tissue engineering using cell sheet technology. Royal Society of Chemistry. 2012;2(6):2184-90.
[3] Haraguchi Yuji, Tatsuya Shimizu, Masayuki Yamato, et al. Regenerative Therapies Using Cell Sheet-Based Tissue Engineering for Cardiac Disease. Aug. 2011;2011:845170-77.
[4] Yamato, Masayuki, Hidekazu Sekine, Joseph Yang, et al. Cell sheet engineering for regenerative medicine: From the viewpoint of inflammation. Institute of Advanced Biomedical Engineering and Science, Tokyo Women's Medical University. 2006;15:156-164.
[5] Nash, Maria E., Deirdre Healy, William M. Carroll, et al. Cell and cell sheet recovery from pNIPAm coatings; motivation and history to present day approaches. Journal of Materials Chemistry. May 2012;22:19376-89.
[6] Elloumi-Hannachi, I., Yamato M, Okano T. Cell sheet engineering: a unique nanotechnology for scaffold-free tissue reconstruction with clinical applications in regenerative medicine. J Intern Med. Jan 2010;267(1):54-70.
[7] Yang J, Yamato M, Shimizu T, et al. Reconstruction of functional tissues with cell sheet engineering. Biomaterials. 2007 Dec;28(34):5033-43.
[8] Burillon, Carole, Laure Huot, Virginie Justin, et al. Cultured Autologous Oral Mucosal Epithelial Cell Sheet (CAOMECS) Transplantation for the Treatment of Corneal Limbal Epithelial Stem Cell Deficiency. Investigation Ophthalmology & Visual Science. March 2012;53:1325-31.
[9] Miyahara, Yoshinori, Noritoshi Nagaya, Masaharu Kataoka, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nature Medicine. Apr. 2006;12:459-455.
[10] Fujita, J., Itabashi Y, Seki T, et al. Myocardial cell sheet therapy and cardiac function. Am J Physiol Heart Circ Physiol. 2012 Nov 15;303(10):1169-82.
