Carticel
Carticel™ , a Genzyme product, is the only autologous arthroscopic cartilage tissue regeneration therapy currently approved by the Food & Drug Administration (FDA) [1].
History
- 1995 – Genzyme releases Carticel™, treats first patient
- 1996 – Genzyme files BLA for Carticel™
- 1997 – Biologics License Application (BLA) approved by the FDA
- This marks the first ever BLA approval for a cell therapy treatment
- 2006 – Good Clinical Practice (GCP) for cartilage repair surgery completed
- 2010 – Carticel™ Identity Assay FDA approved
- This unique assay provides insight into the actual chondrocyte concentration of an individual Carticel culture [1]
Articular Cartilage
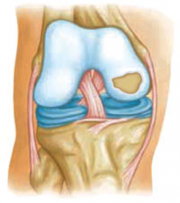
Articular cartilage is comprised of cartilage cells called chondrocytes. This tissue is smooth and slippery, which is essential as it provides a barrier at several bone-bone junctions throughout the body. Along with synovial fluid, articular cartilage absorbs the heavy impacts of walking, running, playing sports, etc. It provides people and animals with ease and range of motion in synovial joints such as the knee, shoulder, hip, ankle, etc.
Damage
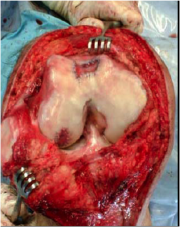
One flaw of articular cartilage is that it can not heal itself "in vivo". Its density and robustness, while essential for its application, deny the tissue the presence of blood vessels. Blood vessels are the key to nutrient transport throughout the body. Without nutrients, damaged chondrocytes can not be healed, and proliferation of chondrocytes is not possible. Areas of articular cartilage damage are called Focal Chondral Legions (FCLs). Causes of FCLs include trauma, wear (especially for people who lead active lifestyles), and osteoarthitis. The latter condition can actually be caused by delaying or neglecting to treat FCLs. FCLs, as shown in Figure 2, expand outward, thus the damage incurred by wear/trauma increases greatly as FCLs are left to grow in size. Osteoarthritis develops when cartilage/bone damage worsens uncontrollably, thus it is clear how FCL growth increases the possibility of this condition. The main symptoms of FCLs are chronic pain, limited joint function, swelling, clicking, locking and catching. Pain and limited range of motion are the main symptoms that are used to diagnose articular cartilage damage prior to an arthroscopic (surgical) diagnosis. 90% of Carticel™ surgeries repair legion(s) no greater than the size of a dime. Legions much greater than this may not be treatable by Carticel™.
Manufacturing
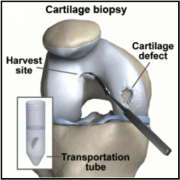
Carticel™ is the final product of autologous chondrocyte culuring in vitro. Once a sample of healthy articular cartilage is obtained from the patient, it is shipped to Genzyme. The sample is in the form of a solid graft, and thus it must be broken down in solution. This solution is then purified, removing synovial cell, as well as ECM and proteins. A graft the size of two tic-tacs can result in a culture of ~12 million cells. This culture can be cryogenically stored for up to 2 years at Genzyme’s facilities. This provides flexibilty for patients and surgeons in scheduling, and can be very important should new complications push back the Carticel™ surgery[1].
Treatment
Since articular cartilage can not repair itself, and FCLs can cause severe pain and lead to osteoarthritis, immediate care is usually very important. Before the release of Carticel™, treatment options for articular cartilage repair were limited to methods, such as microfracture surgery, which require damage to the subchondral bone. This damage induces an inflammatory response (blood flow) which provides the surrounding healthy cartilage with the nutrients it needs to proliferate. The downside to this is that the inflammatory response is excessive, as bone fractures generally take at least a month to heal. Also, the cartilage that forms within the FCL tends to be weaker than the existing healthy tissue [4]. Carticel™ treatment evades the use of bone drilling/fracturing, and provides patients with fresh, healthy chondrocytes. First, the patient’s (healthy) cartilage graft is taken from a minimally weight-bearing section of his/her cartilage. This graft is then handled by Genzyme as discussed in the above (Manufacturing) section. The Carticel™ chondrocyte culture is then injected into the FCL, where it takes the form of the legion. The procedure by which this is executed is called Autologous Chondrocyte Implantation (ACI) [2].
Procedure
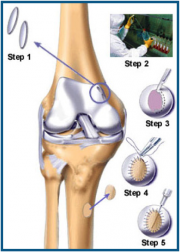
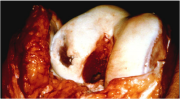
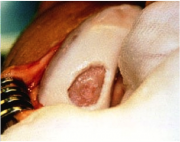
GCP for ACI is very detailed, as is the case for all surgeries. The general flow of the surgery, however, can be outlined in 5 steps (Figure 4). The first step is simply to graft healthy cartilage. This is where the first disadvantage of ACI arises; the total surgery requires two procedures, this step being the purpose of the first procedure. The second step is the actual culturing of Carticel™ by Genzyme. The third step is called debridement. This is basically the removal of unhealthy or dead cartilage from the FCL. This step is essential to ACI because it provides the healthy, cultured chondrocytes a healthy site to attach to. Before (Figure 5) and after (Figure 6) photos show the aesthetic difference made by debridement. Physically, without a debridement step, unhealthy tissue would still not heal, and the weak cartilage around the edges of the FCL would not provide a strong binding site for new chondrocytes. The fourth step of ACI requires a graft of periosteum, shown being taken from the tibia. The periosteum is the skin-like covering of the bone, the second layer of protection. The perosteum graft is cut to the size of the FCL, and stitched around the FCL. It is then checked for water-tightness by injecting saline solution into the created pocket. Figure 7 shows dual periosteum grafts sutured to a large FCL. This fourth step is essential, as the fifth and final step is the injection of Carticel™ into this pocket. Naturally, if any Carticel™ escapes this pocket, positive results are less likely. The new chondrocytes are left to harden and form junctions with the existing healthy cartilage. The following video provides a walkthrough of the Carticel™ treatment process[1].
Rehabilitation
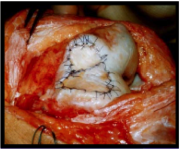
- 4-6 months: Full weight-bearing and low-impact sports (i.e. swimming, stationary bike)
- 8-12 months: High-impact sports (i.e. running)
- 12-18 months: Contact, pivoting sports
- These recommendations are general, based on an average age of ~34 years [1]
Results
- 88% patients reported fair to poor knee condition prior to Carticel™ treatment
- 77% of patients report good to excellent pain reduction
- 49% of patients undergo secondary surgery
- These results based on the pilot study, conducted on patients of an average age of ~34 years, having an average of 1.9 previous procedures [1].
References
- http://www.carticel.com Genzyme’s official product webpage
- http://orthoinfo.aaos.org/topic.cfm?topic=a00422 American Academy of Orthopedic Surgeons' Orthopedic Information site
- http://www.iasm.com/pdfs/Complexcartilageresurfacing.CombinedGenzyme,osteotomy.pdf The Institute for Arthroscopy and Sports Medicine, San Francisco
- http://www.scoi.com Southern California Orthopedic Institute
- Kielpinski, G., Prinzi, S., Duguid, J., and du Moulin, G. (2005). "Roadmap to approval: use of an automated sterility test method as a lot release test for Carticel®, autologous cultured chondrocytes." Cytotherapy, 7(6), 531-541.
- Wang, Y., Ding, C., Wluka, A. E., Davis, S., Ebeling, P. R., Jones, G., and Cicuttini, F. M. (January 2006). "Factors affecting progression of knee cartilage defects in normal subjects over 2 years." Rheumatology, 45(1), 79-84.
- Wood, J. J., Malek, M. A., Frassica, F. J., Polder, J. A., Mohan, A. K., Bloom, E. T., Braun, M. M., and Coté, T. R. (2006). "Auto Cultured Chondrocytes: Adverse Events Reported to the United States Food and Drug Administration." Journal of Bone & Joint Surgery, American Volume, 88(3), 503-507.
