Carmen E. Castaneda: Week 3
From OpenWetWare
Jump to navigationJump to search
Terminology
- Look up the following terms and provide definitions. For each definition, three elements are required: you must quote a source, reference the source, and interpret the definition in your own words.
- dynamical system: A means of describing how one state develops into another state over the course of time.(1) In other words,it is used to describe changes occuring over a time period.
- law of mass action: A law stating that the intensity of a reaction is dependent on the degree of presence of the reactants responsible for the reaction.(3) Which means that its a law which is used to describe the effects of the reation.
- homeostasis: The ability of the body or a cell to seek and maintain a condition of equilibrium or stability within its internal environment when dealing with external changes (2) Meaning that it is what the body, or in our case cells, has to have to be in equilibrium. Maintain a constant internal enviorment, doesn't mean the cell has reached equilibrium but it might be working toward that state. It is the goal of the cell to keep itself balanced, taking into acount everything.
- equilibrium:The state of balance or static; the absence of net tendency to change.(4) We can also say it is the state in which the body is in balance.
- oscillation: Fluctuation; variation; change back and forth.(5) Also said to be a state with a continuous change in between a certain interval.
- autocatalysis: A reaction in which one or more of the products formed acts to catalyze the reaction.(6) This means that the substrates act as catalist and speed up allow more of the reation to occur.
Applying the Law of Mass Action
Construct differential equations that model the following reactions. Be sure to define your state variables and rate constants.
- A + B → C
- Here A and B are the substrates that produce C at a rate of k1 only in a forward direction.
- d[A]/dt = -k1[A]
- d[B]/dt = -k1[B]
- d[C]/dt = k1[A][B]
- A + B ↔ C
- A and B are once again substrates that produce C at a k2 in the forward direction but A and B are also produced from C at a k-1 rate in the backwards direction.
- d[A]/dt = -k2[A]+k-1[C]
- d[B]/dt = -k2[B]+k-1[C]
- d[C]/dt = k2[A][B]-k-1[C]
- A + B ↔ 2C
- A and B are again substrates that produce twice the concentration of C at a k3 in the forward direction but A and B are also produced from twice the concentration C at a k-2 rate in the backwards direction.
- d[A]/dt = -k3[A]+k-2[C]2
- d[B]/dt = -k3[B]+k-2[C]2
- d[C]/dt = k3[A][B]-k-2[C]2
- 2A + 3B ↔ C+D
- With twice the concentration of A and three times the concentration B we see that produce C and D at a k4 in the forward direction but two times the concentration of A and three times the concentration of B are produced by the concentration of C and D in the backwards direction at a k-3
- d[A]/dt = -k4[A]2+k-3[C][D]
- d[B]/dt = -k4[B]3+k-3[C][D]
- d[C]/dt = k4[A]2[B]3-k-3[C]
- d[D]/dt = k4[A]2[B]3-k-3[D]
Please note that the symbol ↔ is used to denote arrows (reactions) in both directions.
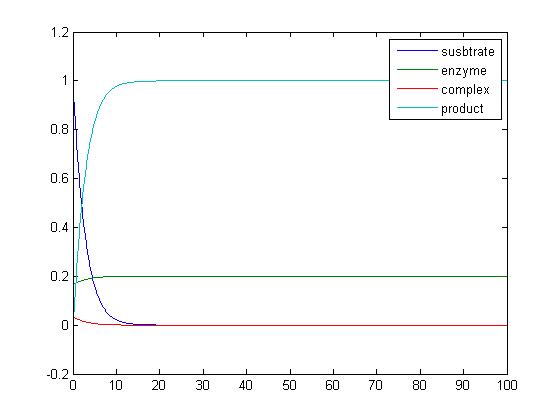
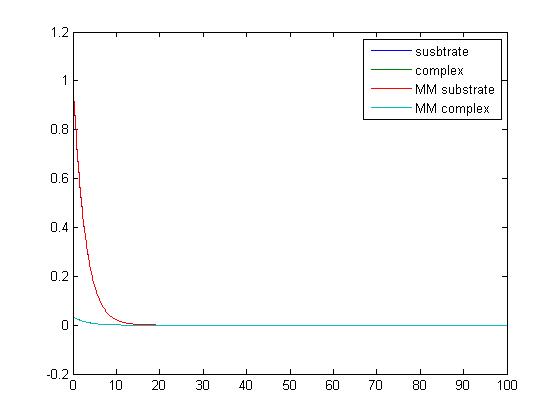
Simulating Reaction Kinetics
- Use the matlab code provided at my lionshare folder to study the simple reaction
- E + S ↔ ES → E + P
that we have studied in class. Set the parameters as follows:
- [S0] = 1.0
- [E0] = 0.2
- [ES0] = 0.0
- [P0] = 0.0
- k1 = 2.0
- k-1 = 0.0
- k2 = 10
Plot the output, and save the plot as an image.