CH391L/S13/CleanGenomes
A clean or minimal genome refers to the minimum set of genes that an organism needs to survive and reproduce in a defined environment. This implies that there are genes that are “nonessential” to the organism’s survival and can be removed without destroying the cell or disrupting its growth cycle. Examples of nonessential DNA would include duplicate genes, transposable elements, regions coding for phenotypic plasticity and catabolic pathways used for the intake and breakdown of complex biomolecules [1][2][3].
Advantages of a Minimal Genome
As the complexity of synthetic biology projects increases, extraneous proteins and other gene products inside cells could interact unfavorably with new synthetic pathways. Minimizing a genome would eliminate the unknown by only including parts with a known function; simplifying synthetic engineering. Listed below are a few of the advantages of using a minimal or reduced genome over a wild-type genome.
1) Improved gene stability Gene stability in reduced genomes has been improved by removing transposable elements (TEs), error prone DNA polymerases and the enzymes responsible for the SOS response. Deleterious DNA (like insertion sequences) can disable genes by inserting themselves unfavorably within the genome. An increase in the number of random genetic changes is extremely harmful toward a minimal cell lacking a number of redundant systems [1][4]. Even cells with a small number of TEs could have up to 3.9% of its genes disabled at any one time [5] . A stable genome is a very desirable trait for research and experiment replication [6][7].
2) Less extraneous gene products Smaller amounts of extraneous gene products, like secondary metabolites, would simplify and reduce the cost of extraction and purification of cell parts, biomolecules and pharmaceuticals, many of which would be more expensive and time consuming to separate from a population of cells by conventional means.[6] Also, production of extraneous metabolites decreases the rate of cellular growth by investing energy into building these products.
Future advances in genome replication, unnatural amino acids, drug development, fuel production, biofilm formation[8] biomaterial synthesis and other cellular applications and processes[7] could be accelerated by simplifying and streamlining the genome used to code for a cell [1][4].
Minimal/Clean Genomes vs. Wild Type Genomes
Cells with reduced genomes have less relative fitness than similar wild type cells and cannot easily cope with changes in its environment. For example, reduced cells derived from E. coli MG1655 are more sensitive to reactive oxygen species, even when the cells still retain the genes needed to respond towards oxidative stress [7].
The mutation rate in cells with minimal genomes is significantly reduced compared to wild type genomes [7]. This would decrease the genetic diversity of the reduced genome cell population. Due to the lack of genetic diversity, it could be plauible that if a bacteriophage ever infected a reduced or minimal genome line, then the cells will be totally destroyed. Otherwise, a reduced mutation rate is a very desirable trait for genome stability.
Estimating the Number of Essential Genes
There are several different methods used for estimating the minimum number of essential genes an organism needs to survive in a controlled environment. Each method has its own shortcomings which limits their applications.
Comparative Genomics
Comparative genomics looks for homologous sequences of DNA between different organisms and strains (Homologous sequences are when two or more DNA strands from different strains or organisms are very similar or homologous). Genetic homology over a wide number of similar organisms could be an indicator of essential genes since they were conserved throughout those strains or species [1][6].
A comparative approach could underestimate the number of essential genes since it only accounts for true genetic orthologs. For example, this gene estimate would not account for genes with different DNA sequneces that code for functionally similar gene products [9][10] . In some instances, it could also overestimate the number of essential genes since homologous genes do not have to be useful or essential. For example, virulence factors that are homologous in many pathogenic microbes are not essential genes [6].
Gene Disruption using Transposable Mutagenesis
Targeted gene disruption using transposable mutagenesis uses transposable elements to inactivate genes. Theoreticlly, if a transposable element is unable to insert itself into a gene, then that gene could be more essential to the cell than other genes that are susceptible to disruption.[11]
Some of the genes screened may read a false positive for essentiality since there is the chance that some transducible elements may not have been transduced into that gene, i.e. a nonessential gene may not have had a transposable element inserted into it. Also, one transposon may disable multiple gene products of varying essentiality (like in alternatively spliced genes). An essential gene could also function normally with a transposable element inside it, which could result in a false negative error [1][9][12] .
mRNA Disruption using Antisense RNA
Antisense RNA (asRNA) is a single strand of RNA that complementary to an mRNA inside a cell. When antisense RNA base pairs with mRNA, the mRNA is unable to be translated. Cells that can't survive in the presence of certain asRNAs indicate that the untranslated gene products bound by asRNA are essential to cellular survival.
Antisense RNA disruption can only work is there is an adequate amount of mRNA to disrupt. For example, intracellular signaling polypeptides may not be targeted by asRNA disruption since they don't need to be highly expressed [9][13].
Genome Reduction
One of the most straightforward approaches to determining a minimal gene set is to reduce the number of genes in a cells genome until it can no longer survive. Reduction would be able to definitively determine the essentiality of trans and cis regions of DNA inside a chromosome. Unfortunately, genome reduction would take a longer time to accomplish since determining a minimal gene set through genome reduction involves, relatively, more trial and error than the other aforementioned methods.
One of the largest reduced, viable cells to date is a modified E. coli MG1655 that has had over 1.8 Mb (38.9%) of it's genome successfully removed [7].
Genome Synthesis
Whole genome assembly is the creation of an artificial genome, with the intent to install that genome into a pre-existing cell. The new genome is assembled in steps, starting with relatively short (~1000bp) sequences that are then assembled into larger and larger pieces. Synthesis of an artificial bacterial genome has been achieved twice: the first was the complete synthesis of the Mycoplasma genitalium genome[14], followed by the complete synthesis (and transplantation into another cell) of M. mycoides [15]. A notable synethic genome that has been planned is M. laboratorium, which will features a reduced genome, and is being worked on by the J. Craig Venter Institute [16].
Genome synthesis has been prohibitive in the past because of its high cost but, fortunately, the cost of DNA synthesis has decreased in the last decade.
Methods for Genome Reduction and Synthesis
Systematic Genome Reduction
One natural approach to engineering strains with a reduced genome is to systematically identify and delete regions of the genome not necessary for host cell survival. Posfai et al. created the MDS strains (multiple deletion strains) by aligning the genomes of multiple genomes of E. coli, identifying regions which were absent in multiple strains, and deleting them via Lambda Red recombination. All IS elements were removed as well, lowering the mutation rate and increasing the stability of genetic constructs introduced into the cell. The strain had comparable growth rate compared to wild type [6][2].
Ara et al. constructed a minimal version of the B. subtilis genome in 2007 [17]. This strain had slightly decreased growth rate compared to wild-type, but displayed normal morphology and similar protein production capabilities.
Iwadate et al. in 2011 were able to use a method involving a red gene-mediated lambda phage homologous recombination system [12] to systematically remove up to 38.9% of E. coli's chromosome [7] .
Explanation of Methods used in Genome Reduction
One method used in 2002 by Kolisnychenko et al. involves the use of site-specific recombination to remove selected DNA from a genome. This method was used to delete DNA fragments anywhere from 7 to 82 kb in length from E. coli MG1655 [6].
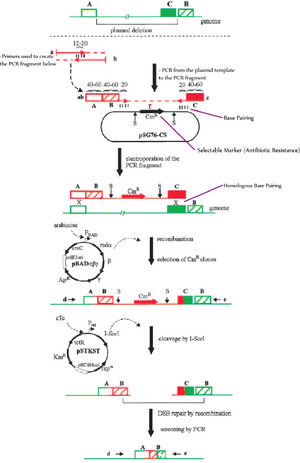
The method used by Ara et al. in 2007 to create a reduced B. subtilis genome was similar to the method used by Kolisnychenko.
Selection for Reduced Genome
Strains of organisms placed under certain conditions are able to be evolved in order to select for a genome of minimal size. Such conditions may include growth in media lacking a wide variety of sugars to favor the loss of biosynthetic pathways or sugar metabolism operons. Growth in structured environments which favor a smaller cell or growth under other conditions which favor the loss of unnecessary genes could also be used to select for a smaller genome size.
A study by Lee et al. removed about 10% of M. extorquens AM1's genome through evolutionary selection by growing it in a controlled environment. The cells were grown on plates containing mixtures of methanol and succinate for 1500 generations. Most of the DNA lost contained accessory genes [18].
Minimal Genome Synthesis
For more details, see: DNA Assembly
Mycoplasma mycoides Synthesis
Gibson et al synthesized the first artificial cell by generating the Mycoplasma mycoides genome from digitized genome information and transforming it into Mycoplasma capricolum cells devoid of genomic information. These cells were capable of continuous self-replication and were identified by "watermarks" inserted in the genome. This technology could be utilized in the future to create cells with novel and useful properties from scratch [14][15]. The same group is allegedly working on a reduced mycoplasma genmome and will probably construct this by using the same genome synthesis techniques used to construct the M. mycoides genome.

iGEM Connection
An iGEM team from Korea created a program to analyze genomes to help find a minimal genome. Their definition of a minimal genome states that a minimal genome is a set of genes that every living organism contains; a comparative genomics approach. They plan on using this software to design their own minimalistic genome.
References
- Forster AC and Church GM. Towards synthesis of a minimal cell. Mol Syst Biol. 2006;2:45. DOI:10.1038/msb4100090 |
- Pósfai G, Plunkett G 3rd, Fehér T, Frisch D, Keil GM, Umenhoffer K, Kolisnychenko V, Stahl B, Sharma SS, de Arruda M, Burland V, Harcum SW, and Blattner FR. Emergent properties of reduced-genome Escherichia coli. Science. 2006 May 19;312(5776):1044-6. DOI:10.1126/science.1126439 |
- Hutchison CA, Peterson SN, Gill SR, Cline RT, White O, Fraser CM, Smith HO, and Venter JC. Global transposon mutagenesis and a minimal Mycoplasma genome. Science. 1999 Dec 10;286(5447):2165-9. DOI:10.1126/science.286.5447.2165 |
- Jewett MC and Forster AC. Update on designing and building minimal cells. Curr Opin Biotechnol. 2010 Oct;21(5):697-703. DOI:10.1016/j.copbio.2010.06.008 |
-
K. Umenhoffer, T. Fehér, G. Balikó, F. Ayaydin, J. Pósfai, F. R Blattner, and G. Pósfai. Reduced evolvability of Escherichia coli MDS42, an IS-less cellular chassis for molecular and synthetic biology applications. Microb Cell Fact. 2010; 9: 38
- Kolisnychenko V, Plunkett G 3rd, Herring CD, Fehér T, Pósfai J, Blattner FR, and Pósfai G. Engineering a reduced Escherichia coli genome. Genome Res. 2002 Apr;12(4):640-7. DOI:10.1101/gr.217202 |
- Iwadate Y, Honda H, Sato H, Hashimoto M, and Kato J. Oxidative stress sensitivity of engineered Escherichia coli cells with a reduced genome. FEMS Microbiol Lett. 2011 Sep;322(1):25-33. DOI:10.1111/j.1574-6968.2011.02331.x |
- May T and Okabe S. Enterobactin is required for biofilm development in reduced-genome Escherichia coli. Environ Microbiol. 2011 Dec;13(12):3149-62. DOI:10.1111/j.1462-2920.2011.02607.x |
- Kobayashi K, Ehrlich SD, Albertini A, Amati G, Andersen KK, Arnaud M, Asai K, Ashikaga S, Aymerich S, Bessieres P, Boland F, Brignell SC, Bron S, Bunai K, Chapuis J, Christiansen LC, Danchin A, Débarbouille M, Dervyn E, Deuerling E, Devine K, Devine SK, Dreesen O, Errington J, Fillinger S, Foster SJ, Fujita Y, Galizzi A, Gardan R, Eschevins C, Fukushima T, Haga K, Harwood CR, Hecker M, Hosoya D, Hullo MF, Kakeshita H, Karamata D, Kasahara Y, Kawamura F, Koga K, Koski P, Kuwana R, Imamura D, Ishimaru M, Ishikawa S, Ishio I, Le Coq D, Masson A, Mauël C, Meima R, Mellado RP, Moir A, Moriya S, Nagakawa E, Nanamiya H, Nakai S, Nygaard P, Ogura M, Ohanan T, O'Reilly M, O'Rourke M, Pragai Z, Pooley HM, Rapoport G, Rawlins JP, Rivas LA, Rivolta C, Sadaie A, Sadaie Y, Sarvas M, Sato T, Saxild HH, Scanlan E, Schumann W, Seegers JF, Sekiguchi J, Sekowska A, Séror SJ, Simon M, Stragier P, Studer R, Takamatsu H, Tanaka T, Takeuchi M, Thomaides HB, Vagner V, van Dijl JM, Watabe K, Wipat A, Yamamoto H, Yamamoto M, Yamamoto Y, Yamane K, Yata K, Yoshida K, Yoshikawa H, Zuber U, and Ogasawara N. Essential Bacillus subtilis genes. Proc Natl Acad Sci U S A. 2003 Apr 15;100(8):4678-83. DOI:10.1073/pnas.0730515100 |
- Lagesen K, Ussery DW, and Wassenaar TM. Genome update: the 1000th genome--a cautionary tale. Microbiology (Reading). 2010 Mar;156(Pt 3):603-608. DOI:10.1099/mic.0.038257-0 |
-
C. M. Trepod,J. E. Mott. Elucidation of Essential and Nonessential Genes in the Haemophilus influenzae Rd Cell Wall Biosynthetic Pathway by Targeted Gene Disruption Antimicrob Agents Chemother. 2005 February; 49(2): 824–826
-
J. Kato, M. Hashimoto. Construction of consecutive deletions of the Escherichia coli chromosome Molecular Systems Biology 3; Article number 132
- Ji Y, Zhang B, Van SF, Horn, Warren P, Woodnutt G, Burnham MK, and Rosenberg M. Identification of critical staphylococcal genes using conditional phenotypes generated by antisense RNA. Science. 2001 Sep 21;293(5538):2266-9. DOI:10.1126/science.1063566 |
- Gibson DG, Benders GA, Andrews-Pfannkoch C, Denisova EA, Baden-Tillson H, Zaveri J, Stockwell TB, Brownley A, Thomas DW, Algire MA, Merryman C, Young L, Noskov VN, Glass JI, Venter JC, Hutchison CA 3rd, and Smith HO. Complete chemical synthesis, assembly, and cloning of a Mycoplasma genitalium genome. Science. 2008 Feb 29;319(5867):1215-20. DOI:10.1126/science.1151721 |
- Gibson DG, Glass JI, Lartigue C, Noskov VN, Chuang RY, Algire MA, Benders GA, Montague MG, Ma L, Moodie MM, Merryman C, Vashee S, Krishnakumar R, Assad-Garcia N, Andrews-Pfannkoch C, Denisova EA, Young L, Qi ZQ, Segall-Shapiro TH, Calvey CH, Parmar PP, Hutchison CA 3rd, Smith HO, and Venter JC. Creation of a bacterial cell controlled by a chemically synthesized genome. Science. 2010 Jul 2;329(5987):52-6. DOI:10.1126/science.1190719 |
- Glass JI, Assad-Garcia N, Alperovich N, Yooseph S, Lewis MR, Maruf M, Hutchison CA 3rd, Smith HO, and Venter JC. Essential genes of a minimal bacterium. Proc Natl Acad Sci U S A. 2006 Jan 10;103(2):425-30. DOI:10.1073/pnas.0510013103 |
- Ara K, Ozaki K, Nakamura K, Yamane K, Sekiguchi J, and Ogasawara N. Bacillus minimum genome factory: effective utilization of microbial genome information. Biotechnol Appl Biochem. 2007 Mar;46(Pt 3):169-78. DOI:10.1042/BA20060111 |
- Lee MC and Marx CJ. Repeated, selection-driven genome reduction of accessory genes in experimental populations. PLoS Genet. 2012;8(5):e1002651. DOI:10.1371/journal.pgen.1002651 |