BioSysBio:abstracts/2007/Kitiyaporn Wittayanarakul
- Add or delete the sections that you require.
On the correlation between drug-resistant pattern of HIV-1 protease inhibitors and binding free energy and structural changes
Author(s): Kitiyaporn Wittayanarakul1, Supot Hannongbua1, Michael Feig2
Affiliations: 1Department of Chemistry, faculty of Science, Chulalongkorn University, Bangkok; Thailand; 2Department of Biochemistry and Molecular Biology and Department of Chemistry, Michigan State University, East Lansing, MI
Contact: mameow50@yahoo.com
Keywords: 'HIV-1 Protease' 'drug-resistance' 'Molecular Dynamics Simulations' 'FDA-drug approve'
Introduction
Protease inhibitor (PR) resistance remains the major limiting in the treatment of HIV infection. Understanding source of mutation at molecular level is a key of success in research and drug discovery. The present studies aim to examine relation between clinical data of drug resistance and the molecular properties obtained from molecular dynamics simulations (MD).
Materials and Methods
MD simulations of 6 FDA-approved drugs, which are Lopinavir (LPV), Ritonavir (RTV), Saquinavir (SQV), Indinavir (IDV), Amprenavir (APV), and Nelfinavir (NFV), were performed using AMBER 8. The total inhibitor-enzyme binding free energy (Gtotal) including entropic contributions of 6 complexes was estimated based on MM-PB(GB)SA approach. The decomposition free energy (GDC), representing interaction between inhibitor and each residue, was calculated and used as the criteria for the mutation prediction.
Results
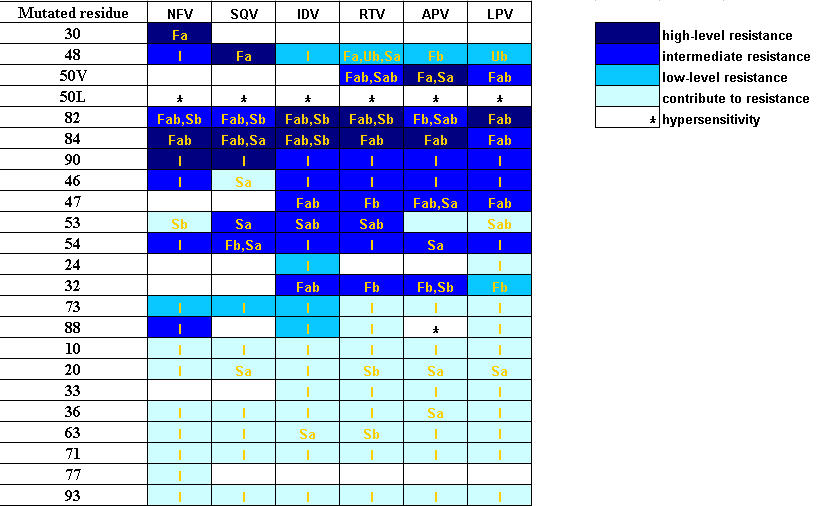
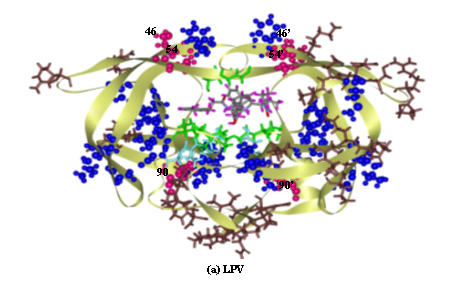
In comparison to the expermental binding free energy, the Gtotal for the 6 complexes yields the standard deviation of ±2.29 kcal/mol, indicating reliability of the method used. To seek for the correlation between resistance according to the clinical data and the molecular properties according to the MD results, the GDC and the structural changes (in term of the root mean square displacement, RMSD, of each residue) were evaluated. For simplicity, the notations F (favourable), U (unfavourable) and S (structure) were, then, used to represent the residue which gives the GDC lower than -0.5 Kcal/mol, the GDC higher than 0.5 kcal/mol and the RMSD higher than 2.0 Ǻ, respectively. In addition, the notation I (indirect) was used to represent the residue which locates close to or between the two residues which have F, U and/or S character, i.e., mutation takes place due to the influence of the F, U and/or S residues. Table 1 contains the mutated residues which correlated with the level of resistance according to the clinical data for 6 drugs. The F, U, S and I characteristic of each residue extracted from the MD trajectories for the 6 complexes were accordingly given. Subscripts a and b indicate its location in chain A and chain B of the HIV-1 PR, respectively. It can be clearly seen that most of the high-level resistance (very dark-blue) takes place at the residues which bind strongly to the inhibitor (F, GDC < -0.5 kcal/mol) and/or residues which have high RMSD (S, RMSD > 2.0 Ǻ). The data shows also that most of the indirect effect, I, effects lead to the low-level resistance (dark blue) and contribute to resistance (light blue). Figure 1 displays location of the residues for the LPV/enzyme complex with different levels of resistances; green for F, light-blue for U, brown for S. The pink and blue CPK represent the mutated residues which are intermediate and contributes to resistance level, respectively. It appears that the mutated residues due to the indirect effect are surrounded by those which have F, U and/or S characters.
Conclusion
Direct correlation between the experimental drug resistance and the calculated binding free energies obtained from the MM-PB(GB)SA as well as the structural changes was observed. Examination was performed for the 6 FDA-approval inhibitors.
Images/Tables
Figure 1.
References
(1) Gohlke, H.; Kiel, C.; Case, D. A. Proc. Natl. Acad. Sci. 1987, 84, 8903-8906. (2) Richard T.; Jonathan M. Special Contribution-Drug Resistance Mutations. 2002, 10, 21-25.