BME100 f2018:Group15 T1030 L6
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | ||||||
OUR COMPANY
FLARTRA LLC LAB 6 WRITE-UPBayesian StatisticsOverview of the Original Diagnosis System In the BME 100 class, 17 teams of similar member amounts (3-6 students) tested patients for the disease-associated SNP utilizing Polymerase Chain Reaction. In total, there were 34 patients, two patients per team. Each of the patients tested was provided with two replicas (making a total of three tubes for each patient) to prevent the instance of error when testing them. Furthermore, there was a negative control tube and a positive control tube provided to be able to compare the data to figure out the correct diagnosis as well as to prevent any other error upon comparing. Errors are avoidable throughout the entire lab, as when taking pictures of the ImageJ calibration controls, the same phone was necessary for taking the same type of photo of each of the droplets, which were taken three times each to get a proper average. They were taken under the same conditions including, but not limited to, height, distance, photo quality, and light control. Additionally, the droplets being used to get a photo of all had the same amount of the given sample and SYBR green in order to further prevent error in the data. Despite the total number of groups, one failed to provide the data, so there were only 32 patients being observed for the class's final data. In addition to the lack of two patients, there was also one patienet with an inconclusive conclusion. Overall, the class was correct in their diagnoses a total of 19 out of 32 times, approximately 59% of the patients. What Bayes Statistics Imply about This Diagnostic Approach For calculation 1 it was very close to 100% which means that the test works. Calculation 1 was to see how reliable our PCR worked for detecting positive for the SNP. For calculation 2 it had the same results as 1 coming close to 100%. Except calculation 2 was to see how reliable our PCR worked for detecting no SNP or the test resulting in negative. For calculation 3 it wasn't even 33% meaning that this test was not very reliable. This calculation was used to see if another positive would come to patients who had risk of developing Parkinson's later in life. Calculation 4 had the opposite results of 3 being almost to 100%. The calculation was used to see if another negative would come from patients who did not have the SNP and it was reliable to be able to tell if the person doesn't have the SNP. Throughout the class lab, there was a great possibility of human error as well as machine error, or even a mix of both. One instance of possible error could include the image-taking process of the droplets; with this came a struggle of getting the same type of photo for each of the droplets. Despite being diligent in the work, a simple mistake could have been made such as the phone being moved and not receiving the same type of photo each time. Another possible error could be during the micropipetting of the liquids. As it had been some people's first time micropipetting (including some in our group), the lack of skill and quick teaching could have resulted in a miscalculated amounts of solution transferred. A last source of error that could have occurred is the preciseness of the ImageJ program. When inspecting the pictures that groups provided it may not be able to read some as well as others due to a difference in the cameras that were used. Intro to Computer-Aided Design3D Modeling Our Design
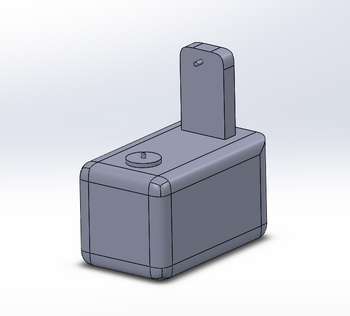
Feature 1: ConsumablesConsumables that would be necessary for our redesigned machine include the use of modified PCR primers. The current primers used in the lab are colorless and are difficult to identify if they are added to the PCR sample or not. the use of a colored primer would eliminate that by having the primers color the solution after they are added. also it would be nice of the primers would change colors after heat was added to the PCR solution, this would indicate if the samples have gone through the full PCR process. currently there is a good product created by BioRad that incorporates the use of colored PCR primers in their PCR system. Also our system would no longer need the use of SYBR Green which would remove issues that have been identified during our run through of the lab. Feature 2: Hardware - PCR Machine & FluorimeterOur improvement is a redesigned fluorimeter. The weakness this redesign is aimed to moderate is having to dye the substance and the time it takes to carefully take pictures of the sample. Our fluorimeter will dye the substance after it is put into the circular compartment and the lid is closed. Our fluorimeter will also take pictures of the sample, so that the use of a phone camera is obsolete.
| ||||||