BME100 f2017:Group2 W1030 L2
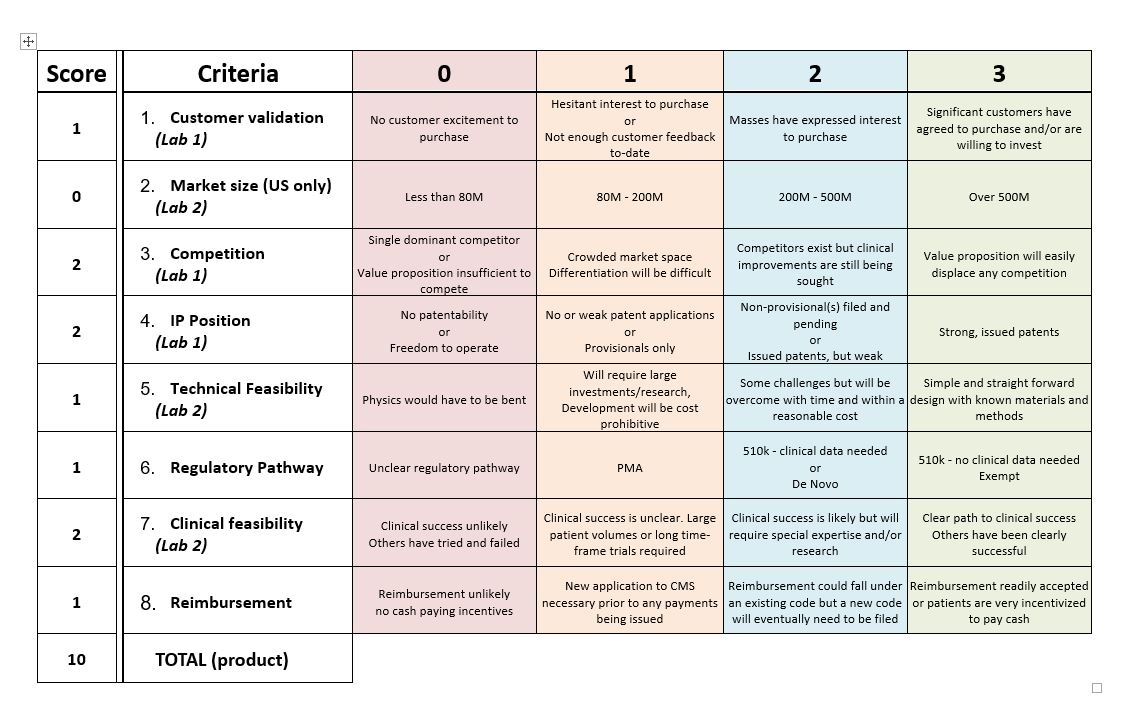
Technical Feasibility
The technologies needed for this product is ultrasonic waves to be transmitted to the brain non invasively as well as the design of the helmet. The challenges of designing this product is going to design this in a way that will make it not only home assessable but comfortable and cost effective. Other challenges this product faces is that we have to make it so that it fits everyone's head since everyone has different head sizes.
Things that could go wrong is that our product just does not work and that our design is a fail which is something that all products face. To our design specifically though, the helmet could just not be a practical way to get ultrasound waves to transmit to the brain and effectively target the subthalamic nucleus and the correct intensity needed.
Clinical feasibility
Our technology is already figured out but we are trying to not only make it more accessible but more portable. The existing technology requires you to go into a clinic and be connected to a machine. Our technology is trying take what is already created and making the treatment be accessible from the comfort of your own home and cut down the cost of the clinic visits.The clinical risk of of ultrasonic stimulation is that you can over excite the brain as well as cause burns to the head. This could in turn require further treatment to heal or adverse the effects of over excitement to the brain. Similar products have been used in clinical trials to rats, mice, and cats. This has been tested in humans with Parkinson's to relieve the symptoms and was successful.
Value
The value that our prototype creates for our customers will be convenience, and cost effectiveness. It will be a simpler solution to treating opiate withdrawals as opposed to the current treatment, which involves many doctors visits, or being prescribed with more opiates. Our prototype will be convenient for the patient because they will not have to take time out of their day to go see a doctor repeatedly. This product will require a minimum amount of doctor’s visits (approximately 2 for setup and detachment, and one for a follow up), and during the setup visit the device will be set to a specific strength so the customer can be confident that there will be minimal issues.
Cost
Market Size: (average 2 million per year X .05 X about $10,000) (with insurance) = $1,000,000,000 per year Or (average 2 million per year X .05 X about $20,000) (without insurance)= $2,000,000,000 per year
Fundability
Though it seems pricey, the cost of this product is significantly lower than the typical visit to the doctors office. To receive ultrasound brain treatment at a facility, the price would have a minimum of tens of thousands (typically $40,000 or higher). Taking into account customer validation, many of those who suffer from opiate addictions would be willing to buy this product. Compared to the competition, the cost of our product is much more affordable and can be done in the comfort of one’s home. Considering these, our product is desirable and fundable.
Sources
https://en.wikipedia.org/wiki/Transcranial_magnetic_stimulation
https://www.fusfoundation.org/diseases-and-conditions/neurological/parkinsons-disease