BME100 f2017:Group14 W0800 L2
TEAM MEMBERS
 |
 |
 |
 |
 |
Our Design
Description of our Device
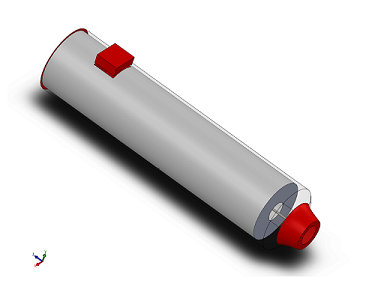
Our device addresses the health issue at hand because it allows for caretakers and/or people to automatically inject a dosage of Lorazepam when one is having a seizure. The device has a slim outer casing to protect the needle from being exposed or contaminated. It device also contains a spring mechanism that triggers the delivery of the drug. The device is built to withstand and be utilized in high-stress situations, so having a switch that acts as a safety forces people to hold the device properly. The Epipen design has confusing indicators on how to hold the device, which results in many people accidentally stabbing their thumb instead of their thigh. This device is more effective than the other treatments, due to the fact that, currently, there are only two drugs used to handle a seizure that has reached an emergency state. The drugs are traditionally administered through rectal insertion or through the use of an IV. The rectal drug is invasive and uncomfortable, and the IV drug is difficult to administer to a patient that is having a seizure. Our device would administer Lorazepam without having to "wrestle" or constraint a patient, which would decrease the risk of incidence when administering treatment. This easy-to-use device prevents injury in an emergency situation by allowing patients to administer the medicine more efficiently by acting as a faster and less invasive alternative.
Technical Feasibility
What are the technologies needed?
- Our auto injector of Lorazepam shares some similarities with the well-known Epipen used to treat anaphylaxis. The device relies heavily on the auto injector mechanism to work properly. The technology required is what delivers a specific and accurate dose of a particular drug, which in this case is Lorazepam. The design requires a spring-loaded syringe to administer the dose. The device will also need latch-type mechanisms to hold the needle and drug in place. The design of our auto injector will allow for easy use and quick relief. The device will also require a switch to operate. The switch will function as a safety, only allowing for the drug to be administered once the switch is manually slid upwards. Unlike the Epipen, our switch will be on the side so people are forced to hold the auto injector the proper way, which would reduce the amount of incidences of people accidentally stabbing their thumb. Our safety mechanism needs to prevent accidental release of a dosage. It should be red when the safety is activated and green once it is slid up to indicate that the drug can be administered. Another necessary component is the inclusion of the technology that registers when the drug has been delivered so that people know when to stop holding the auto injector against their skin. This would be in the form of a visual indication/cue, displaying green once the drug has been delivered. Lastly, we need an outer body mechanism that exposes the needle when the switch is activated and quickly springs back in to cover the needle. Overall, the technology required is not hard to obtain since there are already developed technologies surrounding auto injectors.
What are the challenges?
- The challenges of our device will mostly result from technological obstacles. The biggest challenge is making the shelf life last longer for the drug contained inside of the auto injector. Lorazepam has a shelf life of about 60 days without refrigeration.1 A way to refrigerate the inside of the auto injector would be beneficial, but is difficult to implement in such a small device. Another issue would be with the switch. If the switch does not function properly and allow for the release of the drug, the patient could die. The switch also needs to be accurate when it displays the colors so people know when to inject the drug. Another potential challenge is the visual indication mechanism, which displays when the drug has been fully administered. This is crucial because people need to receive the correct amount of Lorazepam to properly treat their seizure. Lastly, the mechanism behind the spring is essential since it releases the drug. If it does not fire properly, patients will be in trouble. Luckily, these auto injector technologies are not new and there are many successful mechanisms and methods already established.
What could go wrong?
- There are potential issues with our device’s technology, as there is with almost all medical devices. If the mechanisms fail to work, then the drug would not be administered and potential death could result. There are multiple mechanisms that could result in the failure of the drug being administered. For example, if the switching mechanism doesn’t work, then the drug might not be administered. Similarly, if the visual indication fails then the drug might not have been administered effectively and properly. Another factor to note is the shelf life of our auto injector. Without refrigeration, Lorazepam has a shelf life of about 60 days.1 This is crucial information because that is how long the auto injector will remain useful for. Although our design is fairly simple, people may still not know how to use it correctly. In addition to well-engineered technology, we also need to be conscious of the labelling of our device.
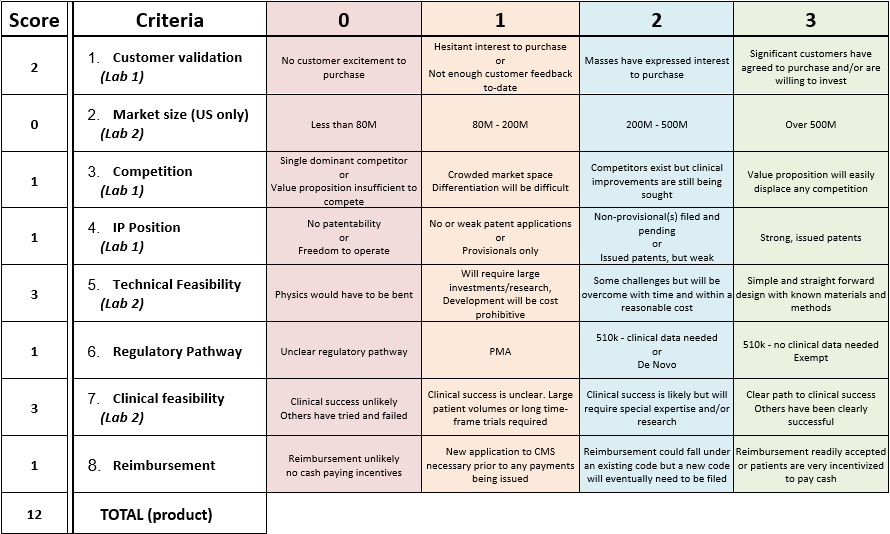
Overall Technical Feasibility: 3
- Based on the fundability worksheet, our device would receive a score of 3 for technical feasibility. This means that our design is simple and straightforward, with known materials and methods. Since there are already established auto-injectors, the technology has already proven to be successful and effective. The materials needed to properly execute the technology have already been established as well. The only mechanism that may be considered questionable is the switch, but other auto injectors usually have some type of safety mechanism involved in their design, so this device should not be to difficult to create.
Clinical Feasibility
Given the technical feasibility, will it work in the clinic?
- Yes, the pen should work in emergency departments and ambulances. The mechanism is simple and is used to administer other drugs, like epinephrine, and is commercialized to the public. Injectable lorazepam is a completely functional way to administer the drug to stop status epilepticus. [10]
What are the clinical risks?
- Accidentally administering too much of the drug and accidentally injecting your thumb with the pen are major clinical risks that come with any device that dispenses medicine that is in the hands of the public. To combat this we have multiple prescribable doses available and a button that you must press on the side to inject the pen. If someone has to hold the button down on the pen, then they will not have their thumb resting on the top of the pen so if it is upside down they will not accidentally inject themselves instead of the person they are trying to help, a common misuse of Epi-pens. This will also help orient the pen so it doesn't go upside down in the first place.
Have similar products been in a clinical trial? How long was the trial?
- Yes, similar products have been tested multiple times with multiple different seizure medications with positive results. Patients who receive the autoinjector seem to heal faster than those who receive the IV.[9]
Overall Clinical Feasibility: 3
- Based on the fundability worksheet our device has a clinical feasibility score of 3. There is a clear path of success based on current emergency procedures dealing with seizure patients. An injection of lorazepam is already used to treat seizures via a syringe, this technology will just make it easier to do so. Research has also been done on the usefulness of a autoinjector device for lorazepam and diazepam and has shown that patients who receive an injection as soon as possible after they start seizing do better post seizure than those who waiting for a professional. [9]
Market Size
As we found previously, there are anywhere from 1.3 million to 2.8 million people with epilepsy.[2] On the professional side, there are roughly 916,000 licensed physicians who may, at some point, need to deal with a seizure.[3] Additionally, there are 5,795 hospitals in the US[4] and 170 specialized epilepsy centers.[5] Even if every physician was equipped with a pen and every major medical facility kept 100 pens, the market size would be below 80 million people. Therefore, our product would warrant a 0 for market size on the Fundability chart.
Fundability
What value does your prototype create for the customer?
- Our product provides non-professionals , as well as physicians and health professionals with an easy-to-use fast response in emergency situations. Additionally, it provides a sense of security for customers dealing with epilepsy in family, or in themselves.
How much does it cost to produce? Why?
- Our product holds a design and material composition comparable to the EpiPen, which, according to Mylan’s CEO, costs $34.50 per pen.[6] Though, compared to the epinephrine dose’s cost of $1,[2] Lorazepam costs approximately $8.80 per dose needed for our product.[7] The cost of production is thus estimated to be $42.30 per pen.
What would be the anticipated average sale price (ASP)?
- Again, using the EpiPen as a reference, a reasonable sale price for our product would be $100 per pen, based off of the EpiPen’s net price of $137.[8] Whereas emergencies requiring EpiPen may not come frequently, epileptic seizures may happen frequently and, depending on the case, may be a recurring expense.
Should it be funded?
References
1. NCBI, "The 60-day temperature-dependent degradation of midazolam and Lorazepam in the prehospital environment". https://www.ncbi.nlm.nih.gov/pubmed/23148574
2. http://time.com/money/4481786/how-much-epipen-costs-to-make/
3. FSMB, "A Census of Actively Licensed Physicians in the United States, 2014" https://www.fsmb.org/Media/Default/PDF/Census/2014census.pdf
4. CDC, "Table 116. Hospitals, beds, and occupancy rates, by type of ownership and size of hospital: United States, selected years 1975–2009". https://www.cdc.gov/nchs/data/hus/2011/116.pdf
5. NCBI, "Data on Specialized Epilepsy Centers: Report to the Institute of Medicine's Committee on the Public Health Dimensions of the Epilepsies". https://www.ncbi.nlm.nih.gov/books/NBK100603/
6. NBC News, "Industry Insiders Estimate EpiPen Costs No More Than $30". https://www.nbcnews.com/business/consumer/industry-insiders-estimate-epipen-costs-no-more-30-n642091
7. Drugs.com, "Lorazepam Prices, Coupons and Patient Assistance Programs". https://www.drugs.com/price-guide/lorazepam
8. Bloomberg, "How EpiPen’s Price Rose and Rose". https://www.bloomberg.com/graphics/2016-epipen-pricing/
9.Salahi, Lara. “Epi-Pen to Stop Epileptic Seizures?” ABC News, ABC News Network, 15 Feb. 2012,abcnews.go.com/Health/Wellness/epi-pen-epileptic-seizures-researchers-step-clos er/story?id=15646106. Accessed 19 Sept. 2017.
10.“Lorazepam Injection : Uses, Side Effects, Interactions, Pictures, Warnings & Dosing.” WebMD, WebMD, www.webmd.com/drugs/2/drug-3953/lorazepam-injection/details. Accessed 20 Sept. 2017.