BME100 f2017:Group10 W0800 L2
Lab 2: Prototype Design
Part 1: Assignment
Our products main function is to be able to regulate the chemicals that the brain makes. We designed our product to do this through electrical stimulation by having our product essentially be a capacitor. The product would contain a high quality insulator on the bottom as well as on top to secure the required amount of charge needed to regulate the chemical production in the brain. The top insulator would also have little tiny conductors so that when the charge is needed to be dispelled it can be released from the capacitor in to the circuitry, then through the nervous system, to the brain. Being able to manipulate the chemical output in the brain would solve many of the neurological problems that many face today such as anxiety and depression. This is the main health issue that we have decided to try to solve with our product.
Technical Feasibility
The overall technical feasibility of our design is very good because of the fact that the technology needed to produce our product has been around for a very long time. The technology needed to make our product would be two insulators and a conductive plate that fits in between. There would also have to be little tiny conductors on one insulator which allows the charge to flow throughout the circuit when needed. The circuit that would be needed would need multiple voltmeters to sense when the capacitor has discharged. The hardest technology to obtain for our product would be a type of metal that would contain a lot of charge, enough to stimulate the brain without being put on the brain, as well as not hurting the patient. The main The technology needed would just be the same basic technology for your generic circuit and capacitor. The biggest challenge would be stimulating the brain chemically without being invasive and as well as letting the electricity flow directly to the brain and not throughout the entire body. Due to the fact that we have not tested our product the chance that something could go wrong is high. One possibility could be that the client could be shocked or given a seizure due to too much electrical stimulation in the brain.
Clinical Feasibility
A)Given the technical feasibility of the design, it should be fairly simple to implement in the clinical setting. However, like mentioned earlier, there is a chance that something may go wrong and potentially harm the patient. An issue that needs to be addressed is how to isolate the charge entering the body so that it only affects the targeted area of the brain and doesn't affect other bodily functions like movement and speech. Another obstacle to overcome is regulating the strength of the charge to the individual needs of the patient. Because this design uses an adhesive to stick to the skin surrounding the spine, it is much less invasive than traditional methods of treatment like surgery.
B) Any time a treatment is conducted that includes deep brain stimulation therapies there are always possible risks that can affect a patient. For instance, the complications in some cases include: Sweating, Nausea, and skin irritation due to the sticky pads. Some of the lesser side effects are, insomnia , shortness of breath, slowing of the heart rate. More complicated side effects include electrical shock, memory loss, and stroke.
C) Have similar products been in a clinical trial? How long was the trial? A similar product for treatment (NCT00662584) called Repetitive Magnetic Transcranial Stimulation was tested in a clinical trial by the University of California in Los Angeles from August 2006 through April 2007. The method consists of applying a non-invasive procedure that uses magnetic fields to stimulate nerve cells in the brain that cause GAD (general anxiety disorders). Currently this product is being used for treatment, but it does not make the symptoms disappear but reduces them and even though it’s non-invasive, it’s not portable since a patient must go to a clinic for treatment and has cost of around $2,500 per session.
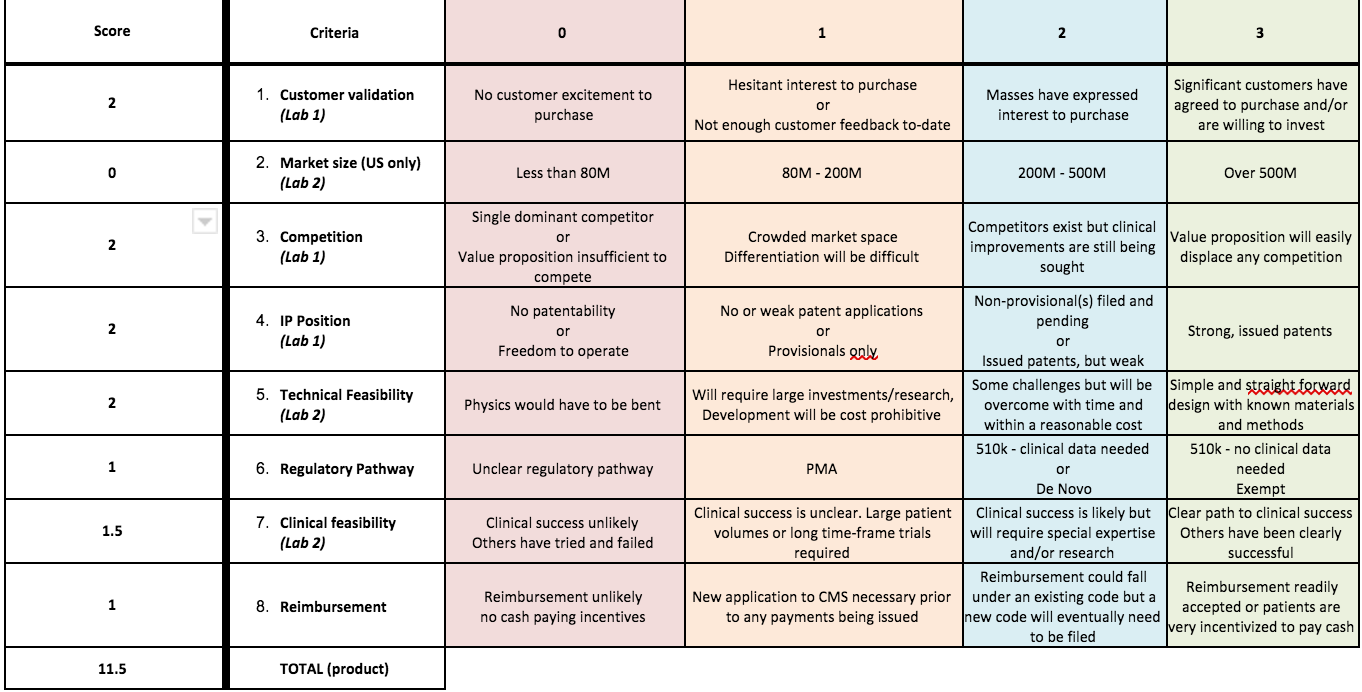
Fundability Worksheet Chart
According to the Fundability Worksheet Chart, our design would score a 2 in technical feasibility. Although most components of our device would be fairly simple to construct, there might be an issue when trying to implement the right type of metal into the conducting system. However, with a bit of research and experimentation, this can be done. As far as clinical feasibility, our device would score between a 1 and 2 because there is a risk that the charge would not be delivered to the designated area of the brain and could potentially harm the patient. Although this could be researched and tested, this could be a timely and costly process. Additionally, in order to correctly use this device, the clinician should have a clear understanding of circuits and voltage and be able to identify potential risks the patient might have regarding this type of treatment.
Part 2: Assignment
1. Our product provides an easily accessible and convenient route to managing anxiety and depression-like systems. Because it is so small ( 1 inch X 1/2 inch X 1/10 inch) and goes on with an adhesive strip, it is easy to hide and doesn't interfere with the patient's daily activities.
2.Determine the Cost to Create Your Design.
The over all cost to create our design would be at least ten dollars. This is the amount we came up mathematically because of the fact that the cost of the circuitry throughout the product was around $3.63. Our product would need at least three times the amount of circuitry which would make the cost around ten dollars. We came up with this number by using a certain amount of silver in our product for conductivity and calculating the cost that way(1).This does not account for the material that we would need to store the insane amount of charge. We can not determine the cost amount of this material because we do not know if it exists or not.
3.What is the Average Sale Price? The average sales price would be around $50 because of the fact that we wanted it to cost at least five times the amount to make it.
4. According to data collected by Anxiety Association and Depression of America, 18.1% of the population in the USA suffer from anxiety disorders(2). That will be equivalently to around 58,481,100 people in USA suffering anxiety disorders. The market size in dollars will be around $146,202,750. (58,481,100 population with anxiety disorders x 0.05 assumed market penetrance x $50.00 predicted cost of product)
5. Using the fundability worksheet, determine if your prototype should be funded. Justify why or why not. Using the fundability worksheet it can be reached the conclusion that the prototype should be funded because even though it has a small market size and low clinical feasibility since no clinical trial of a similar product from a competitor has been yet made, this product has a good customer validation, technical feasibility, competitors exist but are still improving, and the biggest base is that anxiety disorders are becoming very common around people in the US meaning that the market size will continue increasing or remain the same after people who used our device have controlled symptoms and are removed from the current market size. (3)
Resources
1). Gleason, Stefan. “Silver Spot Prices Per Ounce Today, Live Bullion Price Chart USD.” Money Metals Exchange, Money Metals Exchange, 9 Aug. 2017, www.moneymetals.com/precious-metals-charts/silver-price.
2)“Facts & Statistics.” Anxiety and Depression Association of America, ADAA, 7 Apr. 2016,
adaa.org/about-adaa/press-room/facts-statistics. Accessed 16 Sept. 2017.
3)“Repetitive Magnetic Transcranial Stimulation (RTMS) in the Treatment of Generalized Anxiety Disorder (GAD).” Repetitive Magnetic Transcranial Stimulation (RTMS) in the Treatment of Generalized Anxiety Disorder (GAD) - Full Text View - ClinicalTrials.Gov, 19 June 2017, clinicaltrials.gov/ct2/show/NCT00662584?term=non%2Binvasive&cond=Anxiety%2BDisorder&cntry1=NA%3AUS&draw=1&rank=1. Accessed 15 Sept. 2017.