BME100 f2015:Group3 1030amL1
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
OUR TEAM
LAB 1 WRITE-UPIndependent and Dependent VariablesIndependent Variable: The independent variable for this study is the dosage of Lipopolysaccharide in milligrams. This is the independent variable because the dosages will be controlled and administered with the intent to find the smallest dosage of Lipopolysaccharide that will be able to increase Inflammotin levels in the elderly. Dependent Variable: The dependent variable is the level of Inflammotin found in the subjects. This is the dependent variable because it is dependent on the dosage of Lipopolysaccharide in the subjects system. This is the variable that is being examined in order to evaluate the effects of varying levels of Lipopolysaccharide. Experimental DesignSubjects will be processed through a 12 week experimentation. Each group will be made up of a different age ranges, including 7 males and 7 females, with a total of 6 groups. This means a total of 84 subjects: 42 male, 42 female. These groups will be randomly selected within their age group and gender. Each group will be ingesting pills of lipopolysaccharide to increase the amount of a newly discovered inflammatory protein (Inflammotin) in the elderly. To gain a baseline reading for the first week subjects will be given a 0mg baseline placebo test to see their baseline levels of Inflammotin. After each week of testing, subjects will take a week off of testing to re-balance their bodily systems. A weekly regimen includes a pill taken at 9am daily followed by testing of Inflammotin levels at 9am (daily baseline), 12pm, 3pm, and 6pm. Each blood sample test will be taken using the ELISA method. Test results will be averaged to see the daily effect. After each 2 week period (1 week of testing followed by 1 week of rest), subject groups will start a new, randomly chosen dosage to avoid subjects building tolerance and keep the trials random. Concluding testing results will be averaged due to multiple tests in each age group; trends in levels of Inflammotin per age group will be examined.
Null Hypothesis - There is no correlation between age and effective dosage of agent. Because the controlled groups in this experiment represent the alteration of a second independent variable (age), it must be statistically proven that this variable plays no role in determining the smallest effective dosage. To do so, the results from each dosage must be analyzed for variance individually using an ANOVA test. If the mean measurements of Inflammotin levels for each age range are similar enough, it can be determined that there is no correlation between age and dosage required and the data can be said to be representative of the entire population tested. Using the information from the “Rotation Schedule Chart”, variation within each age group can be found using the Sum of Squares Within (SSW) as well as the variation between age groups using the Sum of Squares Between (SSB). These two values can then be used in conjunction to create an F-statistic to be compared to a critical F value in order to determine if the null hypothesis is true or not. When F-Statistic > the Critical F Value, the null hypothesis is false.
When F-Statistic < the Critical F Value, the null hypothesis is true.
If the null hypothesis is true we can assume that the means gathered from the recorded data in the “Rotation Schedule” chart are viable and true for patients of all ages. If the null hypothesis is false and there exists a high variation in the results between age groups, then the data must be approached more in depth to find the lowest effective dosage for each age group.
Subject SelectionA noticeable age deviation in the United States begins at the age group of 60-64. Past that age range, the population of the elderly begins to significantly decrease as illustrated in the graph below. Therefore, subjects between the ages of 60-89 are the most desirable for this experiment. Past the age of 89, the population dwindles down too much and would interfere with the progress of the study too much, because it would be difficult to find healthy, suitable, and willing participants to begin the study. 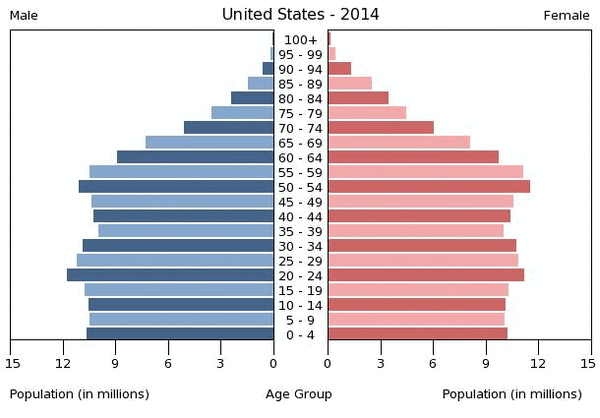 The primary control of this experiment would be the age groups and number of subjects per group selected. The age ranges for the elderly subjected for this study will be from ages 60-89 years old. The experiment will be conducted with a total sample size of 84 subjects, 42 male and 42 female with 7 males and 7 females per group. Here is a table illustrating the specified group assignments:
Subjects will be selected as diversely as possible, from different locations, with few underlying medical conditions and prescriptions that could potentially affect the efficacy of the drug. No subjects selected may be within a 20 mile radius of other participants; this is to ensure that there will be randomization and no bias in the experiment. This experiment will not take in those who have chronic inflammatory issues or other severe medical conditions that would affect the individual subject from participating in the duration of the 12-week study.
Sources of Error and BiasThere are certain variables that can affect the results of this experiment. The subjects are being selected from as large of a population as possible in order to ensure diversity. However, having such a diverse group of test subjects could make the data more varied and less accurate. Additionally, the lab has a low budget, which could also affect how and what kind of participants are gathered for the experiment. If more resources were available, further testing might show the effects of the drug on different ethnicities. Some of the test subjects may also be using medications that could alter the effects of the drug being tested. It is highly unlikely that the people being tested will not be taking any other medications besides lipopolysacchride during the experiment. People between the ages of 60-89 develop more medical problems and are therefore more likely to be taking regular doses of medications. A subject's medical history might cause certain side effects while taking the drug. The lifestyle choices of a subject are essentially impossible to control. Diet and exercise can change depending on age. Some of the younger subjects may be more physically active than those in the age range of 80-89. As people age, many tend to neglect their diets and become less active due to mobility problems. Some of the subjects may also be more deficient in inflammotin than others, in which case they may need a higher dosage of the medication. The severity of each subject's condition can cause a higher variance in the results of the experiment. Tolerance can also cause variance. Some may be more affected by the drug than others while others can receive a higher dosage with no effects.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
