BME100 f2015:Group17 8amL6
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | |||||||
|
OUR COMPANY
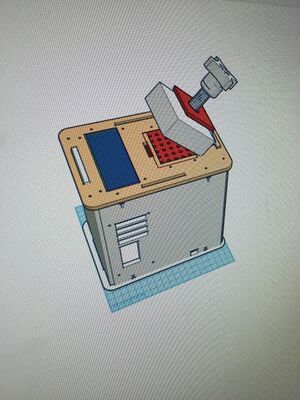
LAB 6 WRITE-UPBayesian StatisticsOverview of the Original Diagnosis System PCR reactions require great care and precision in order to get quality data. A group of 34 students were separated into groups of 6 students, therefore making 17 groups. Each group had DNA samples from 2 patients, 3 for each patient. The 3 samples for each patient would ensure the most accurate results. The micropipette gave extremely accurate measurements of all the solution samples. The ImageJ calibration made sure that the right area of the drop was selected for the photograph. The best results were when the ImageJ encompassed the drop and wasn't affected by light on the outer materials. In order to test the reliability of the PCR data, Bayesian statistics were performed. The first calculation tested the probability of having a positive final test conclusion given a positive PCR result. The second calculation tested the probability of having a negative final test conclusion given a negative PCR result. The third calculation tests the probability that the patient will develop the disease, given that the final test conclusion is positive. The fourth calculation tests the probability that the patient will develop the disease, given that the final test conclusion is positive. What Bayes Statistics Imply about This Diagnostic Approach The probability of getting a positive final test conclusion is a 29% chance. The probability of getting a positive PCR reaction is true 31% of the time. The probability of having a positive PCR reaction given a positive final test conclusion is 92%. The probability of having a positive final test conclusion given a positive PCR result is 86%. The probability of getting a negative final test conclusion is 57% for the trials. The probability of getting a negative diagnostic signal is a 46% chance. The probability of having a negative diagnostic signal given a negative final test conclusion is equivalent to 77%. The probability of having a negative final test conclusion given a negative diagnostic signal is 95%. According to the cumulative data, the probability that the patient will develop the disease is %32. The probability that the final test conclusion is positive is 29%. The probability that the final test conclusion is positive, given that that the patient will develop the disease is a 25% chance. The probability that the patient will develop the disease, given that the final test conclusion is positive is a slim 28% chance. The probability that the patient will not develop the disease is a 68%. The probability the final test conclusion is negative is true for 57% of the trials.The probability the final test conclusion is negative, given that that the patient will not develop the disease has a probability of 69%. The probability that the patient will not develop the disease, given that the final test conclusion is negative is very probable, at 82%. One Source of error could be the amount of light that made it into the box where the fluorimeter was set up. This would add external light as opposed to just having the light from the fluorimeter be the only light source. Another possible source of error could be derived from the Image J program. In the process of Image J there is a part where after splitting the color channels, an oval is drawn over the drop to measure the amount of SYBR Green in the sample. In this step of the process it is possible to measure a glimmer of light that could skew the data in a certain way. One last source of error is having the phone camera too far from the fluorimeter and also throwing off the data. Intro to Computer-Aided DesignTinkerCAD The TinkerCAD tool is a quick way to 3-D model objects and inventions and is easy to understand. An important feature of TinkerCAD was having the ability to import 3-D designs from the Thingiverse website. It made the process of structuring the PCR machine much easier. TinkerCAD is similar to Solid Works in many different ways. Solid works has an important mechanism that allows two different parts to be mated together at precise points. This feature is critical because it can correctly piece multiple parts into an assembly. TinkerCAD lacks in this way because the only means of mating two or more separate pieces together is by maneuvering with the mouse which may not always be accurate. However, TinkerCAD makes it easier to rotate and move different pieces as well as create cuts and holes whereas Solid Works involves many different coordinates and angle measures in order to perform simple actions. It may be more precise and better quality to use SolidWorks but TinkerCAD is definitely the quicker and less invasive option. Our Design Entire Product Close Up
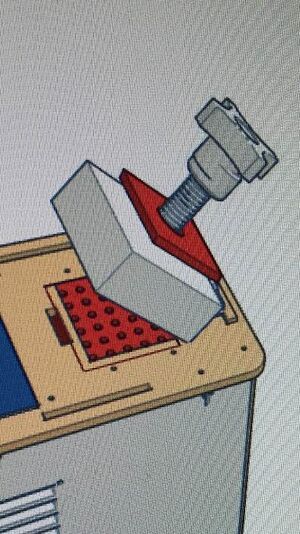
Our additions to the PCR design have been highlighted in red. The first component that we decided to adjust was the lid stopper. Sometimes when loading samples into the Open PCR and closing the lid, the lid will be tightened too much onto the samples and cause the heating plate to corrupt the samples and throw data off in the future. With our design, we create a plate that acts as a stopper for the lid so that it prevents tightening samples too much. This would assure that the data is not corrupted from the lid. Our second edition was simply expanding the number of samples that can be tested in one Open PCR run. We were able to add significantly more samples so that there would not have to be waiting to add samples into the OpenPCR as much. This makes the new process much more efficient than how it was before.
Feature 1: ConsumablesThe consumables that will be packaged in our kit include : plastic tubes and tips, slides, pipettors,PCR mix and primers. The number of the consumables can be designed at any company since we are not changing them. However, our design will be able to hold about 20 extra test-tubes and the holding part is also modified so that it can hold the tubes, just right and not too tight. Therefore, the plastic-tubes should be manufactured from the same company that will manufacture the PCR machine. Also since the PCR machine can hold up to 20 extra plastic tubes,the Kit will contain more test-tubes equivalent to the additional test-tubes.
Feature 2: Hardware - PCR Machine & FluorimeterThe changes made to the product were only external, so the hardware remains the same from the original design. This hardware includes a fan, LCD, and circuit board. Additionally, the new design also includes the same handle, heating lid, heater, and stopper. All of these parts are the same size and design.
| |||||||