BISC220/S10: Mod 3 Lab 10
The Effects Of Cytochalasin B On The Actin Cytoskeleton
The Cytoskeleton
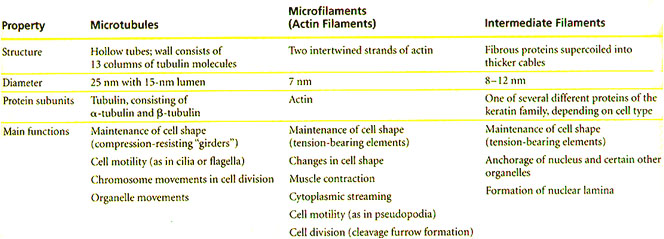
The cytoskeleton contains an intracellular array of protein fibers that functions to maintain or change the shape of cells, to anchor organelles, to contribute to cell motility, and to aid in cell division. There are three types of protein fibers in the cytoskeleton of the eukaryotic cell: microfilaments, microtubules and intermediate filaments. These fibers are distinguished by their size and subunit composition. As summarized in Table I, microfilaments are polymers of actin, microtubules are polymers of α- and β-tubulin heterodimers, and intermediate filaments are composed of several different classes of proteins.
Table 1. Structure and Function of the Cytoskeleton
The three components of the cytoskeleton. The specific proteins, the size and the functions of each of the fibers are delineated. (Adapted from Campbell, 2006).
These cytosolic fibers are not just static bundles of proteins. The cytoskeleton is a dynamic network within the cell that is constantly changing to accommodate various cell processes such as motility, vesicle transport, or cell division. Anything that disrupts the dynamic nature of one of the cytoskeletal components will adversely affect the cellular processes that depend upon this component. Wang et al. (1998) have found that exposure to cytoskeleton-interfering agents may result in the activation of stress induced signaling pathways that can lead to apoptosis in some cell types. Schafer et al. (1998) found that treating fibroblast cells with various chemicals that prevent actin polymerization inhibited cell motility.
Pinocytosis
Pinocytosis and phagocytosis are the two main components of endocytosis – the process by which cells take up particles and solutes outside the cell and internalize them. The difference between the two processes has to do with particle size. Phagocytosis, or cell eating, takes up particles greater than 250 nm in size. Pinocytosis, or cell drinking, takes up fluid and solutes into vesicles about 100 nm in size.
Pinocytosis usually begins at regions on the cell membrane called clathrin-coated pits. These regions of membrane continually invaginate into the cell and pinch off to form clathrin-coated vesicles. Extracellular fluid is taken up into these vesicles as they form, resulting in both the fluid and membrane becoming internal. A second mechanism for pinocytosis involves formation of caveolae, deep invaginations in the cell membrane composed of structural proteins called caveolins. The lipid composition of the caveolae is thought to determine the “cargo” carried, while the protein dynamin is required for pinching off the caveolae.
Fluorescent Labeling of Actin Filaments:
Fluorescence microscopy enables the visualization of proteins or other cellular components that have been labeled with specific fluorescent probes within intact cells. Fluorescent molecules absorb light at one wavelength and emit light at a longer wavelength. If a fluorescent dye is illuminated at the proper wavelength and then viewed through a filter that allows only the emitted wavelength of light to pass through, the molecule will glow against a dark background. The probes used for fluorescence microscopy can be antibodies, other proteins or peptides, or small molecules that bind specifically to the targeted cellular components. These probes can either be directly conjugated (or covalently linked) to a fluorescent dye, or the fluorescent dye can be conjugated to a second molecule that binds to the probe. The first strategy is referred to as directly labeling, whereas the second is called indirect labeling. Once the probe has been incubated with the cells and excess probe has been washed away, the labeled cells can then be viewed through a fluorescence microscope. The observation of fluorescence indicates the presence and location of the target molecule within the cell.
You will use a direct fluorescence labeling technique to visualize actin filaments within your cytochalasin B treated and untreated mouse fibroblast cells. The probe you will use to label the actin is a molecule called phalloidin, which is derived from the toxic Amanita phalloides (“Angel of Death”) mushroom. This molecule binds tightly to the interface between subunits of actin filaments (F-actin), locking these subunits together and stabilizing the filaments against depolymerization. Phalloidin can bind to actin filaments in the cells of many types of animals and plants, but it does not bind to monomeric actin (G-actin). If the phalloidin is linked to a fluorescent dye, it becomes a very useful probe for localizing actin in cells. You will use phalloidin that has been conjugated to the fluorescent dye rhodamine, which emits fluorescence in the red range of the spectrum. Pinocytotic vesicles will be visualized using Lucifer Yellow as a tracking dye.
Before you label your cells with the rhodamine-phalloidin, you will allow them to “drink” Lucifer yellow by forming pinocytotic vesicles and caveolae. You will then expose some cells to a stronger or a weaker concentration of cytochalasin B while others are unexposed to the drug. You will then fix and permeabilize all the cells. Fixation kills the cells and creates covalent crosslinks between neighboring molecules in the cells, thus “locking” them into place, whereas permeabilization alters the cell membranes to allow the fluorescent probe to access its target molecules. A variety of chemicals can be used for fixation and permeabilization; choosing which of these reagents to use is often critical for the success of a fluorescence labeling assay, and the optimum combination must be determined empirically. In this case, you will use formaldehyde as a fixative and acetone as a permeabilization reagent. In addition to labeling the actin filaments with the red fluorescent phalloidin probe, you may see pinocytotic vesicles stained with a yellow fluorescent dye, Lucifer yellow. You will also be able to visualize the cell nucleus with a stain for DNA: 4,6-diamidino-2-phenylindole (DAPI), a blue fluorescent molecule that binds between the bases of the DNA helix. The DAPI is a component of mounting medium that you will apply to your slides at the end of the labeling procedure. The DAPI staining will allow you to locate the nuclei in your cells when you observe them under the fluorescence microscope.
Protocol
Microsoft Word File: Media:Cell Staining Protocols.doc
In today’s lab, you will examine the effects on your mouse fibroblast tissue culture cells of cytochalasin B, a drug that specifically targets the actin cytoskeleton. In particular, you will assess the viability and the integrity of the actin cytoskeleton in cells treated with different concentrations of the drug. The lab will be divided into groups, and each group will test two concentrations of cytochalasin B in the range 0-50 µM. Untreated cultures will serve as controls. After the drug treatment, you will prepare cells for fluorescence microscopy to visualize the intracellular actin filaments.
NOTE #1: Gloves should be worn throughout the treatment, fixation and labeling procedures.
NOTE #2: All steps of the protocol are carried out at room temperature unless otherwise indicated.
Making your working dilutions of the stock cytochalasin B
Your group will be provided with a 50 µM stock solution of cytochalasin B in cell culture medium to dilute with sterile medium (not with PBS!) to the concentrations you are assigned to test. The drug concentration range we will test is 0-50 µM.
Devise a strategy for diluting the cytochalasin B stock solution to obtain your 2 assigned concentrations: either 5 and 50µM or 2 and 20μM. You will need a total of 3 ml of the cytocholasin B concentration you are testing. Check your calculations with your instructor before proceeding. After making the dilutions, mix well.
A. Treatment of 3T3 Fibroblast Cells with Lucifer Yellow and Cytochalasin B for Phallodin Staining of Actin
- Your cells have been incubating with Lucifer Yellow (0.5 mg/ml) for approximately 1 hour to allow for uptake of the dye by pinocytosis.
- Well 1: No Lucifer Yellow; NO cytocholasin B – negative control
- Well 2: Lucifer Yellow; NO cytocholasin B – control for Lucifer Yellow
- Well 3: Lucifer Yellow; concentrated cytocholasin B
- Well 4: Lucifer Yellow; dilute cytocholasin B
- When your cytochalasin B dilutions are prepared, take a growth chamber slide out of the incubator. Empty the media from the chambers into the waste beaker provided. Save the Petri dish with wet filter paper for later use as a humidity chamber.
- Label the slide with pencil or crayon with your initials or some other identifying information. Into the 2 slide growth chamber wells closest to the frosted labeling area of both slides, aseptically add 1 ml of sterile cell culture media (no drug). The fibroblasts in these wells will be your control cells.
- Into the 3rd well of each slide aseptically add 1 ml of the more concentrated cytochalasin B.
- Place 1 ml of the less concentrated drug into the last well of the slide.
- Draw a diagram of the slides in your lab notebook noting the concentration of the drug in each well.
- After the drug or control medium has been added, place the slide back in the humidity chamber Petri dish, wet the filter paper with your squirt bottle of distilled water, put a piece of your group’s color tape on the top of the dish, and return it to the incubator at 37°C with 5% CO2 for 20 minutes. Be sure to record the exact amount of time that the fibroblast cells incubate with the drug.
B. Finishing the Rhodamine-phalloidin Staining of Actin Filaments
- At the end of the drug incubation period, discard the media from the slide growth chamber into the waste beaker near the sink.
- Remove and carefully rinse the chamber divider in distilled water. Set it aside for later use. (Your instructor will help you with this step.)
- At your bench, make sure your slide label is still clear and visible. If not, use a graphite pencil or waxed crayon only to improve it. Place the slide in a Coplin jar filled with PBS for 3 minutes. Move the slide into another jar containing fresh PBS for a second 3-minute wash. Repeat 2 more times for a total of 4 fresh PBS washes.
- Wearing gloves, remove the slide from the PBS and place it in a Coplin jar in the hood containing 3.7% formaldehyde in PBS for 10 minutes to fix the cells.
- Using forceps, remove the slide from the formaldehyde and wash in a Coplin jar of fresh PBS for 3 minutes, repeat two more times for a total of 3 x 3- minute washes. The washing (and the rest of the procedure) may be done at your bench.
- Remove the slide from the PBS. Touch the edge to a paper towel to drain excess buffer and place it in cold acetone in the -20° C freezer for 3 minutes to make the cells more permeable.
- Remove the slide from the acetone, air dry briefly (30 seconds - 1 minute). Place the slide in fresh PBS for 3 minutes. Repeat the washing 2 more times with PBS for a total of 3 x 3 minute washes. Drain excess PBS off the slides by touching the edge to a paper towel before proceeding to the labeling procedure.
- Place the slide into your humidity chamber and moisten the filter paper if necessary. Place the chamber divider back onto your slide in the original position so that the 4 numbered areas are separated.
- Obtain the Rhodamine phalloidin (0.5 µg/ml) from your instructor. Gloves must be worn when handling phalloidin since it is a toxin.
- Using a micropipet, pipet 100 microliters of 1% BSA in PBS into one of the wells containing untreated fibroblast cells. These cells will serve as the negative control for the experiment. Pipet 100 microliters of rhodamine phalloidin onto each of the remaining three wells. Be sure to record which wells contain your control and which contain the probe. Cover the humidity chamber with the top of the petri dish, place foil over the chamber to block out all light, and incubate for 20-25 minutes. Do not move the slide or the chamber during the incubation period.
- Remove slide from the humidity chamber and remove the chamber divider but save the divider. Immediately wash the slide by placing it in a Coplin jar of fresh PBS for 3 minutes and repeat three more times for a total of 4 washes. Rinse your chamber divider in PBS and place it on a paper towel to save it.
- Drain excess PBS off the slide by touching the side of the slide to a paper towel. Place 1-2 drops of mounting medium with DAPI (1.5 µg/ml) on each well of the slide. Cover with a long coverslip. Seal around the coverslip with clear nail polish to make sure it doesn’t move and the slide doesn’t dry out.
- Place the labeled slide into a cardboard slide holder. The mounting medium should preserve the fluorescence for approximately two weeks. Your instructor will store the class slides in the refrigerator until the next lab period.
For lab next week:
During the next laboratory period, each group will view their slide preparation using a fluorescence microscope and obtain photographs to be included in an abbreviated lab report, which will be due the last week of classes. Also remember that, in preparation for the presentations during the last lab session, your group must find a research article that uses your chosen imaging technique to investigate the cytoskeleton. Bring this to next week’s lab session to be approved by your instructor.
References:
Alberts, Bruce et al. (2002) Molecular Biology of the Cell, 4th ed. Garland Publishing Inc., New York.
Campbell, Neil A (2002) Biology 6th ed. Benjamin/Cummings Publishing Co., San Francisco.
Forscher P and Smith S J. (1988) Actions of cytochalasins on the organization of actin filaments and microtubules in neuronal growth cone. The Journal of Cell Biology 109: 1505-1516.
Haggarty SJ, MayerTU, Miyamoto DT, Fathi R, King RW, Mitchison TJ, Schreiber SL (2000) Dissecting cellular processes using small molecules that perturb mitosis. Chem Biol 7: 275-286.
Lodish, Harvey et al. (2004) Molecular Cell Biology, 5th ed. W.H. Freeman and Co., New York.
Mordacq, John C, Ellington, RobertaW. (1996) Immunofluorescence of Cytoskeletal Proteins. Proceedings for the 17th Workshop/Conference of the Association for Biology Laboratory Education 17: 85-97.
Rao S, Orr G, Chaudhary A, Kingston D, and Horwitz S. (1995) Characterization of the taxol binding site on the microtuble. The Journal of Biological Chemistry 270: 2035-2038.
Schafer D, Welch M, Macheskey L, Bridgman P, Meyer S, Cooper J. (1998) Visualization and molecular analysis of actin assembly in living cells. The Journal of Cell Biology 143: 1919-1930.
Toyama,Sakuji and Toyama, Sumi. (1988) Functional Alterations in ’-actin from a KB cell mutant resistant to cytochalasin B. The Journal of Cell Biology 107: 1499-1504.
Wang, T, Wang H, Ichiho H, Giannakakou P. Foster J, Fojo T, Wimalasena J. (1998) Microtubule-interfering agents activate c-Jun N-terminal kinase/stress-activated protein kinase through both Ras and apoptosis signal regulating kinase pathways. The Journal of Biological Chemistry 273: 4928-4936
Useful Web Sites:
http://www.probes.com/handbook/sections/1101.html
http://biology.berkeley.edu/crl/flow_cytometry_basic.htm
http://www.home.eznet.net/~webtent/colchicine.html
http://www.missouri.edu/~chemrg/210w97/taxol_bodypage.htm