BISC110: Series 3 Experiment 10 Hill Reaction Variables
Lab 10 INVESTIGATION OF FACTORS AFFECTING THE HILL REACTION
Introduction
In this lab, you and your partner will explore, in a self-designed, controlled experiment, one factor that may affect the rate of the Hill reaction. The following are some of the factors that you may choose to investigate:
1. Temperature
Consider the normal growing conditions which spinach usually tolerates. How would varying the temperature between 0°C and 65°C affect the rate of the Hill reaction? Would the temperature itself affect the behavior of DCPIP? In terms of the practical aspect of this experiment there are several things to think about: you will need to equilibrate each tube for a few minutes at each temperature in a water bath before adding the thylakoids; you will have to dry the test tube between readings; therefore, you may want to read the absorbance every 20s or 30s instead of every 15s.
2. Illuminance
In our study, we are interested in knowing how much light is falling on our sample, rather than how much light is coming out of the lamp. Furthermore, it is more accurate to use the term illuminance here in place of light intensity (luminance or luminous intensity) because we are able to measure illuminance with our light meters. Illuminance is defined as the light incident or falling on a surface, whereas light intensity is a measure of the rate at which light is emitted in a given direction from a light source. The SI (Systéme Internationale) unit for illuminance is called lux, while the candela is the unit for light intensity.
According to the inverse square law illumination decreases not in proportion to the distance from the source, but in proportion to the square of the distance.
I = intensity in candelas
d = distance in meters of target surface from the emitting source
Would you expect a linear increase in electron transport rates with increasing illuminance? Why/why not?
You can vary the light output of the fiber-optic illuminator (hence, the illuminance on your sample) by varying the setting of the intensity knob. Your instructor will demonstrate how to measure illuminance using a handheld light meter.
3. Light Wavelength
Based on your knowledge of the pigments that are involved in absorbing light for photosynthesis, how would the wavelength of light affect the rate of the Hill reaction?
You can vary the wavelength of light by covering the fiber-optic light pipe lens with colored (red, green, blue) polycarbonate filters (Roscolux®). Make sure to keep the illuminance constant as you vary the wavelength. Measure the % transmittance spectra of the colored filters (Appendix C, Program #6) to get a better idea of their efficacy as wavelength-selective light filters. To save time, you may do this while you are preparing your thylakoid extract.
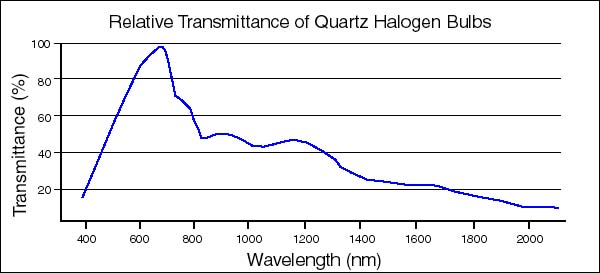
Figure 1. Relative Transmittance of Quartz Halogen Tungsten Lamps. The 150-watt projection lamps of our fiber-optic illuminators have this type of spectral energy distribution curve. The transmittance peak (%Tmax) is 675nm.
4. Inhibitors/Uncouplers
The inhibitors act either by binding to a component of the membrane or altering membrane structure by denaturation and/or solubilization. If the electron transport is blocked, or the membrane is disrupted, there will be a decrease in electron transport and in the reduction of DCPIP. For inhibitors, the effect is concentration-dependent. For chemicals altering membrane structure, inhibition is usually time-dependent as well as concentration-dependent.
The uncouplers act by "uncoupling" electron transport from its rate-limiting dependence on the photophosphorylation machinery. Uncouplers may have various mechanisms of action, but always, the end result is the dissipation of the proton gradient. We cannot measure the production of ATP, but we can measure electron transport using the dye, DCPIP. When the reactions relating to electron transport and the creation of an H+ gradient are uncoupled, electron transport proceeds at a faster rate. Therefore, the reduction and therefore color change of DCPIP occurs faster.
We shall supply stock solutions of several compounds that act as inhibitors and/or uncouplers of electron transport depending upon their concentrations:
2.0 M NH4Cl (ammonium chloride)
10–3 M DCMU (dichlorophenyldimethylurea)
(Note that DCMU can irritate the eyes, skin and respiratory tract. It is used as an herbicide, so it is advisable to wear gloves when handling DCMU.)
5% Triton-X 100 (a detergent)
You may want to dilute the stock solutions of these compounds to find the concentration at which they no longer affect the Hill reaction. Remember that some compounds may act as uncouplers or inhibitors depending on their concentration.
Procedure
Make table(s) and flow charts for your self-designed and instructor approved protocol in your lab notebook. Be sure you have controls and that all of the final volumes of your reaction tubes are the same. Calculate how to make any dilutions before you come to lab. Inhibitors and uncouplers are usually added in 100µL aliquots to insure that the concentrations of all of the other ingredients in the reaction tubes are altered as little as possible. Calculate the effective concentrations of your inhibitor or uncoupler (the concentration while the reaction is occurring). Set up a table in which you can keep track of the times vs. the absorbance for each reaction.
Laboratory Cleanup
- Clean all glassware, centrifuge tubes and pipettes as in Lab 9.
- Dispose of any hazardous waste (reactions containing DCMU) in the container in the hood.
- Other assay reactions can be discarded in the sink.
- Place test tubes in the glass disposal container.
- Return the color filters to the instructor’s bench.
Assignment
1. Plot your A580 data (y-axis) versus time in seconds (x-axis) for the different treatments (you can plot several or all data sets on one graph). Plot all data on the same graph as regression lines for ease of comparison (Appendix E). Include the regression equations. The slopes represent the rates of the Hill reactions. Do not include points that have leveled off. Calculate the rates (change over time of the linear portion of your graphs) and plot those values against your variable (x-axis).
2. A complete scientific research report on your experiment today is due at beginning of Lab 11. Refer to the Resources section of the Lab wiki for general information on scientific writing. It is important that you discuss your results in terms of how the factor that you chose to investigate affects the electron transport rate and the coupling of electron transport with the photophosphorylation of ADP and/or the role of the H+ gradient in these processes. Please compare your results to other investigations published in the primary literature as part of the Discussion section of your report. Refer to the " Guidelines for science writing" information found in the Resources section of the wiki.