Axel:Schwekendiek Working with bacterial stocks
A bacterial culture long-term culture is made by inoculating bacteria into a vial containing LB agar with the appropriate antibiotic. After overnight incubation, bacterial growth should be visible both in the puncture and on the surface of the agar. I recommend the following protocol (taken from the Addgene website) for recovering plasmids. This protocol includes how to isolate a single colony from your stab culture, how to recover plasmid DNA, and how to make glycerol stocks for long-term storage.
Isolating a Single Colony
- Obtain an LB agar plate with the appropriate antibiotic.
- Using a sterile pipette tip, touch the bacteria growing within the punctured area of the stab culture. (A sterilized wire loop or sterile toothpick can be used in place of a sterile pipette tip.)
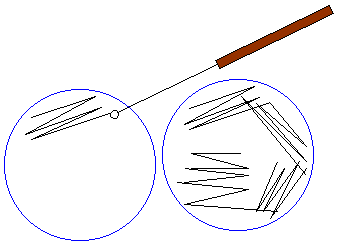
- Run this tip lightly over a section of the plate, as shown in the figure, to create streak #1.
- Using another sterile pipette tip, pass through streak #1 and spread the bacteria over a second section of the plate, to create streak #2.
- Using a third sterile pipette tip, pass through streak #2 and spread the bacteria over the last section of the plate, to create streak #3.
- Grow overnight in a 37°C incubator (unless a different growth temperature is indicated on the plasmid datasheet).
- In the morning, single colonies should be visible. If the bacterial growth is too dense, re-streak onto a new agar plate to obtain single colonies.
Recovering plasmid DNA
- Prepare liquid LB with the appropriate antibiotic. For minipreps, many people use 3 mL of culture, but we use 5 mL.
- Using a sterile pipette tip, touch a single colony of bacteria from your agar plate.
- Inoculate the liquid LB by swirling the tip in it.
- Grow bacterial culture for ~16 hours in a 37°C shaking incubator (unless a different growth temperature is indicated on the plasmid datasheet).
- In the morning, bacterial growth should be visible. Centrifuge the culture to pellet the bacterial cells, then proceed with DNA preparation. Many companies (such as Qiagen) sell kits for isolating plasmid DNA. We use the Fermentas GeneJet Miniprep Kit.
Making glycerol stock for long-term storage
- Follow steps 1-4 under "Recovering plasmid DNA".
- The optimal concentration of glycerol for long-term storage is unknown. Most labs store bacteria in 15-25% glycerol. As an example, a 25% glycerol stock can be made by adding 500 μL of an overnight culture to 500 μL of 50% glycerol in a 2 mL screw top tube.
- Freeze the glycerol stock tube at -80°C in a storage box labeled with your team number and team name.
Recipes
Luria Broth (LB) (500 mL): This recipe will make 500 mL of LB media.
1. Weigh ingredients below and add to 500 mL of distilled water in a bottle:
5 g Tryptone
2.5 g Yeast extract
5 g NaCl
500 mL H2O
2. Cap bottle, but do not tighten.
3. Autoclave using liquid cycle.
4. Tighten lid and store at room temperature.
Luria agar plates (50): This recipe will make about 50 plates (100 mm diameter).
1. Weigh ingredients below and add to 1L of distilled water in a 2L flask:
10 g Tryptone
5 g Yeast extract
10 g NaCl
15 g Agar
1 L H2O
2. Cover flask tightly with foil.
3. Autoclave using liquid cycle, then cool to 55°C.
4. Add appropriate volume of antibiotic. (For example, add 1 mL of a 100 mg/mL ampicillin stock to obtain a final concentration of 100 μg/mL).
5. Pour a thin layer of LB agar into each petri dish (about 20 mL), and cover with lid immediately.
6. Let plates cool for a few hours or overnight.
7. Store plates in plastic bag at 4°C.
50% glycerol (500 mL):
1. Add 250 mL 100% glycerol to 250 mL distilled water in a bottle. 2. Cap bottle, but do not tighten. 3. Autoclave using liquid cycle. 4. Tighten lid and store at room temperature.