20.109(S08):Preparing cells for analysis (Day4)
Introduction
Today you will start collecting the key data for your chondrocyte phenotype experiment. Recall that chondrocytes may de-differentiate to fibroblasts if not kept in the appropriate environment. So, how do we tell these two cell types apart?
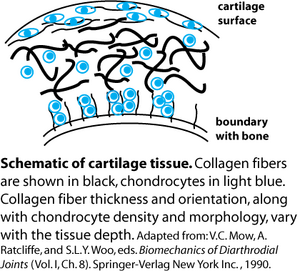
Folks trying to engineer cartilage tissue have been in interested in this and similar questions for some time. After all, the more closely an in vitro or in vivo model construct can mimic natural tissue and promote its development, the more successful it may be for wound and disease repair. Engineering tissue thus requires an expert understanding of what the native tissue is like. Articular cartilage is a water-swollen protein network consisting of >50% collagen Type II, along with small amounts of collagen Types IX and XI. The collagen fibrils vary in diameter, cross-linking density, and orientation (random or aligned) depending on the depth of the tissue cross-section that is examined (see figure). Unlike cartilage, many other connective tissues are composed primarily of collagen Type I.
Extracellular matrix (ECM) proteins such as the collagens must be synthesized by cells. Chondrocytes readily synthesize collagen II, while fibroblasts primarily synthesize collagen I. Thus, the expression and production of different collagens is one way to distinguish these two cells types. To study collagen at the gene transcript level, you will break open and homogenize your cells using a lysis reagent and column (QIAshredder) and then isolate RNA using an RNeasy kit from Qiagen. This kit uses silica gel columns that selectively bind RNA (but not DNA) that is >200 bp long under appropriate buffer conditions. Due to size exclusion, the resultant RNA is somewhat enriched in mRNAs relative to rRNA and tRNA. To further purify for mRNA, one could use a polyT affinity column to capture the polyA tail of this RNA type, but we will not do this today.
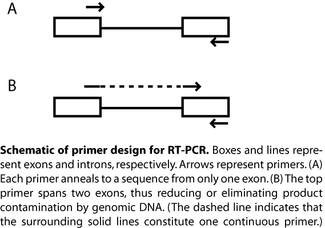
After eluting and measuring your total RNA, you will perform RT-PCR (the RT here stands for reverse transcription) to turn the mRNA into cDNA and amplify the gene transcripts of interest, namely those for the collagen I and collagen II alpha chains. We will use a 1-step RT-PCR kit from Qiagen, though the two procedures (reverse transcription and PCR) can be performed separately. The Qiagen kit utilizes a cocktail of two different RT enzymes: Omniscript and Sensiscript, the latter optimized to detect very low abundance transcripts. After reverse transcription, these enzymes are inactivated, and a heat-sensitive polymerase is activated so PCR can begin. For RT-PCR, primer design must be appropriate for a cDNA rather than genomic DNA. For example, a single primer that includes sequence from two neighbouring exons (along with a second primer that has sequence from just one exon) will amplify mRNA but not genomic DNA, which may be present as a contaminant (see also figure). What will happen if each primer contains sequence from only one exon?
Next time you will run the amplified cDNA products out on an agarose gel, and compare the collagen II:I ratios for your three different culture conditions. (Recall that a high ratio indicates a more chondrocyte-like phenotype.) As you may have noticed by now, agarose gels – not to mention our polaroid camera and scanner – do not have a large dynamic range. In an ideal world (perhaps a future iteration of this module!), we would want to use a more sensitive method for quantifying the transcripts. To get more quantitative and reliable results, one can use real-time-PCR, sometimes called RT-PCR, confusingly! The method is also called q-PCR, for quantitative-PCR. In q-PCR, the amount of DNA (often a cDNA, as in RT-PCR) is measured after each cycle of PCR. This is done by using a dye that fluoresces only when it binds to DNA (similar to ethidium bromide staining), or even a tagged primer that fluoresces only when it binds to the desired product. Several of the fluorophores available exploit FRET, or fluorescence resonance energy transfer, between two molecules. As the DNA is amplified, fluorescence is repeatedly measured and increases exponentially over time. Finally, cDNA product renaturing competes with primer annealing and the fluorescence intensity plateaus rather than growing. Comparisons between samples are done using data in the exponential regime.
Data analysis for q-PCR can be complicated, and we won’t go into all the details in this course. However, even for end-point RT-PCR, one semi-quantitative technique that can be used to compare transcripts from different samples is normalization with a housekeeping gene. That is, one simultaneously amplifies the cDNA of interest and a cDNA for a protein such as GAPDH. This is similar to running a loading control on a gel, but trickier! The primers for the two genes must be compatible – e.g., they must not hybridize with each other. Moreover, the primer amount must be carefully optimized such that the housekeeping gene (high abundance) isn’t totally saturated when the gene of interest (often low abundance) isn’t even readable on the gel. Finally, ideally the housekeeping gene should have a similar amplification efficiency to the gene of interest.
Next time (day 5) we’ll initiate an assay called ELISA to observe collagen at the protein (rather than transcript) level and also begin analysis of the data collected thus far. On day 6, we will discuss image-based analysis in some detail, and briefly consider other distinguishing features of cartilage tissue.
Protocols
If you got to go to the TC room first on Day 2, you will go in the second cohort today (and vice-versa).
Part 1: Prepare cell lysates
The cultured chondrocytes may have secreted some collagen protein into the surrounding medium. In order to test this, save the supernatants from your cells today!! The cells themselves will then be split into two groups: one for RNA isolation, one for protein assay.
Before proceeding, read Part 3 of today's protocol and decide how you want to process your 3D samples.
- Follow the Day 3 procedure (trypsin for monolayer culture, EDTA-citrate for alginate culture) to recover your chondrocytes from each sample and count them.
- Remember to save 1 mL of each cell supernatant into an eppendorf tube – this step should happen prior to rinsing the cells with PBS.
- When you are ready, give your supernatants to the teaching faculty to be frozen until next time.
- Try to minimize the time your cells spend in trypsin, to retain as much protein at their surfaces as possible.
- Count your cells as on Day 3, at a 9:1 ratio with Trypan blue. Note down the approximate numbers of live and of dead cells. Talk to the teaching faculty if you have < 4 M (million) live cells total.
- Set aside ~ 2M cells for RNA isolation in an eppendorf tube, which you will pellet back in the main lab (10 min at 250 g).
- Set aside an equal number of cells from each of the samples for protein assay. (For example, if one sample has 3M cells leftover, and one has 4M cells, take only 3M cells from each.) Pellet them in the main lab as well, then hand them to the teaching faculty to be frozen.
- Before pelleting your cells, clean your microfuge. You can finish setting up your RNA work area while the cells spin down.
Part 2: RNA isolation and measurement
Today you will isolate RNA from your cells, to test for collagen message. RNA is strikingly different from DNA in its stability. Consequently it is more difficult to work with RNA in the lab. It is not the techniques themselves that are difficult; indeed, many of the manipulations will seem identical to those used for DNA. However, RNA is rapidly and easily degraded by RNases that exist everywhere. There are several rules for working with RNA. They will improve your chances of success. Please follow them all.
- Use warm water on a paper towel to wash lab equipment, like microfuges, before you begin your experiment. Then wipe them down with “RNase-away” solution.
- Wear gloves when you are touching anything that will touch your RNA.
- Change your gloves often.
- Before you begin your experiment clean your work area, removing all clutter. Wipe down the benchtop with warm water then “RNase-away,” and then lay down a fresh piece of benchpaper.
- Use RNA-dedicated solutions and if possible RNA-dedicated pipetmen.
- Start a new box of pipet tips and label their lid “RNA ONLY.”
Qiagen sells a kit for isolating RNA and we will be using their protocol and reagents. Remember to balance your three tubes with appropriate symmetry. Also, label your samples carefully at every step.
- In the fume hood, add 1.5 μL of β-mercaptoethanol to 1.5 mL of RLT buffer.
- Remove the supernatant from your cell pellets using pipet tips from your fresh tip box. (Discard in a conical tube.)
- Now add 350 μL RLT-β per cell sample – vortex or pipet to mix.
- Add each cell lysate to a separate QIAshredder column, which is used to remove particulate matter. Microfuge the columns (over a collection tube) for 2 min at max speed.
- Add 1 volume (slightly > 350 μL) of 70% ethanol to each lysate and pipet to mix.
- Apply each sample (including any precipitate) to a separate RNeasy mini column (over a tube). Microfuge for 15 sec and discard the flowthrough.
- Add 700 μL RW1 buffer to each column. Microfuge 15 sec and discard the eluant again.
- Transfer the columns to fresh collection tubes. Then add 500 μL RPE buffer atop the columns, microfuge as before (15 sec), and discard the flowthrough.
- Repeat the addition of 500 μL RPE, but this time centrifuge for 2 min. prior to discarding the flowthrough.
- Place the columns on fresh collection tubes, and centrifuge for 1 min. Running a column like this helps to fully dry it, and to prevent carryover of ethanol.
- Trim the caps off of three new 1.5 ml eppendorf tubes (save the caps!) and label the sides of the tubes.
- Transfer the dried columns into the trimmed eppendorf tubes and elute the RNA from the columns by adding 50 μL of RNase-free water to each. Microfuge for 1 min then cap the tubes and store the eluants on ice.
- Measure the concentration of your RNA samples by adding 5 μL of of each to 495 μL sterile water. (The water does not have to be RNase-free since the RNA can be degraded and still give legitimate readings in the spectrophotometer.) Make your dilutions in an eppendorf tube and use your P1000 to transfer the dilution to a quartz cuvette. Measure the absorbance at 260 nm. Water in one of the optically paired cuvettes should be used to blank the spectrophotometer, but if another group has done this already, it does not have to be repeated.
- A few things to be aware of when using quartz cuvettes:
- They are very expensive.
- The lab has only one set.
- When you are done using the cuvette, you should carefully clean it by shaking out the contents into the sink and rinsing it once with 70% EtOH, then two times with water. Quartz cuvettes get most of their chips and cracks when someone is shaking out the contents since it is so easy for the cuvette to slip from wet fingers or be hit against the sink. Don’t let this happen to you.
- A few things to be aware of when using quartz cuvettes:
- Note the RNA concentrations of your samples in the table below, using the fact that 40 μg/mL of RNA will give a reading of A260 = 1.
- Ideally, you will use 200 ng of RNA in each RT-PCR reaction. However, you also want all reactions to start with an equal amount of RNA template. Moreover, you cannot add more than 30 μL of template per reaction. If you can use 200 ng per reaction within these contraints, do so. Otherwise, figure out which one of your samples is limiting (has the least RNA), and scale all the other sample amounts that you add so they are equal. The table below may be helpful.
| Sample | A260 | RNA conc. (μg/mL) | Max RNA (ng in 30 μL) | Volume RNA needed |
|---|---|---|---|---|
| 2D | ||||
| 3D-1 | ||||
| 3D-2 |
Part 3: Pepsin digestion of protein fraction
If you have the time and inclination, you can digest your alginate beads with pepsin to improve protein recovery. Pepsin is an enzyme that can solubilize collagen.
Per 5 beads, you should use 137.5 μL of EDTA-citrate/acetic acid/pepsin solution. Set the beads aside in an eppendorf tube, and leave them soaking in the pepsin solution overnight at 4 °C. Tomorrow we will move them to the freezer.
To treat your samples more comparably to each other, you can also resuspend your 2D cell pellet in the same pepsin solution.
Part 4: RT-PCR
- The thermal cycler will be preheated to 50 °C while you prepare your samples. This is required for the procedure to work optimally.
- Set up your reactions on a cold block as usual. You will prepare two reactions for each of your samples: one to amplify collagen I, and one for collagen II. You will also run two control reactions, to ensure that there is no contamination or problem with the primers. These will lack template RNA. Thus, you are running 8 reactions total, 4 per collagen type.
- Retrieve ~95 μL of Master Mixes I and II from the teaching faculty. These contains water, buffer, dNTPS, primers, and and an enzyme mixture.
- Now you can add the appropriate RNA template to each tube, according to the calculations you performed in Part 2. Be sure to do one collagen I and one collagen II reaction for each sample. Also, prepare your two template-free controls.
- You should first add a mixture of template and water that has a volume of 30 μL total to each PCR tube.
- Then add 20 μL of the appropriate Master Mix. Pipet the MasterMix prior to use to mix it well.
- The following thermal cycler program will be used:
| Segment | Cycles | Temperature (° C) | Time | Purpose |
|---|---|---|---|---|
| 1 | 1 | 50 | 30 min | reverse transcription |
| 2 | 1 | 95 | 15 min | activate polymerase, deactivate RT enzymes, denature template |
| 3 | 35 | 94 | 1 min | denature (PCR) |
| 55 | 1 min | anneal (PCR) | ||
| 72 | 1 min | extend (PCR) | ||
| 4 | 1 | 72 | 10 min | final extension |
After the RT-PCR is completed, the teaching faculty will store the samples in the freezer until next time.
In whatever time remains today, you can start working on reviewing your lab partner's essay, continue discussion of your shared research idea, get started your cell viability analysis (see day 5), etc.
For next time
- Continue to develop your research proposal and its presentation. (Nothing for this will be collected next time.)
- Exchange rough drafts of your essays with your lab partner. Begin by reading the tips for peer review from Neal Lerner. Then, as you read your lab partner's writing, be a thoughtful and sensitive reviewer. You will do your partner a disservice if you say the writing is great when it's not. However, you may intimidate and insult your partner by writing comments that are too harsh or too obscure (or simply too many!) to be integrated into a revision. The scheme described below may help you to organize your response.
- Start with the positive! Mention one or two things your partner did really well: these strengths could relate to the organization, the writing style, a clever argument, or something else that strikes you. Next, try to restate the main idea of the essay in a sentence or two – this will give your partner a sense of the clarity of his or her writing. Go on to briefly restate the supporting evidence for this idea, and remark on its value and clarity. (Sample comments: The way this evidence relates to your main argument is only hinted at – can you make it more explicit?; This statement seems to contradict your main point – can you reconcile them?; This technical term should probably be defined for your audience.) Finally you should comment on the writing itself: is the tone engaging? are there transitions between ideas that serve the flow of the argument? are there confusing/awkward sentences that need re-writing? Try to give specific suggestions for improvement whenever possible. Print out two copies of your review, one to hand in and one for your lab partner.
Reagent list
RT-PCR Master Mixes:
| Componenet | Concentration | Volume |
|---|---|---|
| Primers | 0.6 μM each | 1.5 μL of 1:5 dilution each |
| dNTPs | 400 μM each | 2 μL |
| Enzymes | unknown | 2 μL |
| Reaction buffer | N/A (multi-component) | 10 μL |
| Water | N/A | 3μL |
| Template(+more water) | 1pg-2μg | Added by students |