20.109(F12): Mod 3 Day 2 Phage nanowires
Phage:SWNTs:TiO2 & Phage:gold:TiO2 nanocomposites
Introduction
Eventually, we'll build a solar cell where the photoanode is made from the material that we're synthesizing today. By templating the assembly of the nanotubes-TiO2 nanocrystal with the M13 phage, the electronic properties of the photoanode are improved.
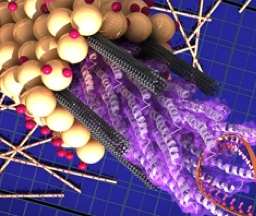
"Improved" here means a few things. Engineers trying to optimize photovoltaic devices want lots of high mobility electrons inside their devices since these can more efficiently convert the photo-energy input to electrical power output. Single-walled nanotubes made from carbon are one kind of material that ranks pretty high for this property. Engineers would also like to optimize the "sink" for these mobile electrons since the very best photovoltaic devices will also efficiently collect them where they're needed. In our experiment, we are relying on M13 to facilitate electron paths by arranging the SWNTs into a higher-order architecture, thereby improving their properties. Not only can M13 bundle the SWNTS so they don't clump together(see how the p8 proteins allow the SWNTs to associate in parallel to the phage in the photo), but the viruses also position parts of the SWNTs to the surrounding solution, allowing for a more complete coating with TiO2. The TiO2 nanocrystal shell (like its more expensive silicon cousin) is needed to pass the electrons from the photo-excited dye...which we'll be adding later.
Note, however, that SWNTs aren't the perfect material, even when arranged by M13. The SWNTs aren't homogeneous and the metallic contaminants can short circuit the electron's paths. For this reason, you are testing different ratios of SWNTs to phage and building solar cells from these variants. You will identify the most useful ratio at the end of the module when you measure the photon-to-current conversion efficiency (IPCE) for the 1:1, 2.5:1 and 5:1 ratio (SWNTs:phage), and we compare across groups.
Today in lab you will react your SWNT:phage with titanium isopropoxide, harvest a small aliquot to visualize with TEM next time, then wash the remainder of the nanowires several times, first with ethanol, then with water. You will have time during these steps to work on the research proposal idea you've got started with your lab partner.
Protocols
Part 1: React SWNTs:phage with Ti(I-pro)4
Today's lab has some safety hazards and you must work extremely carefully. Lab coats, gloves and goggles are a must when you're at the chemical hood. The reaction of the complexed phage with the titanium will take place in the hood at supercooled temperatures (a bath at ~ -40°C). Once the titanium has been deposited on the surface of the phage, the solution is less hazardous, though you should still treat the materials with care since no reactions run to completion
For SWNT phage only:
- Retrieve your dialyzed samples.
- Carefully transfer the contents of the dialysis bag to a 15 ml falcon tube. This is best done by carefully holding the dialysis bag vertically while you remove one of the alligator clips, cover the open end of the tubing with the falcon tube, then invert the tubing and the falcon tube so the complexed phage solution can be collected.
- If there are any nanowires remaining in the dialysis tube after you have emptied the contents, rinse the nanowires out using 1 ml of the NaCl dialysis buffer and add the volume to the 15 ml falcon tube.
- Use the markings on the tube to estimate the volume of the nanowires.
- Vortex the nanowires if they look clumpy.

For all groups:
- Chill your complexed phage on ice on your bench until you are ready to react it with the titanium.
- Calculate the amount of material necessary for making TiO2 Nano composites. The reaction should take place in a 95% ethanol solution. You have two choices:
- a. If your phage volume is less than 250 uL total. You may simply use 5 ml of 95% ethanol.
- b. If your phage volume is > 250 uL total, calculate how much 100% ethanol you would need to add to achieve a 95% solution. Assume that your phage solution is 100% water.
- Add ethanol volume from a. or b. without phage to a 250 mL erlenmyer flask with a stir bar and set aside.
- Prepare a super-cooled bath:
- Mix ethylene glycol and ethanol in a 1:1 volume ratio (make up a total of 100 mL).
- Place the 250ml Erlenmeyer flask with your ethanol solution + stir bar inside an evaporation dish in the fume hood on a stir plate.
- Fill the evaporation dish with the ethylene glycol and ethanol mixture match the liquid level inside the Erlenmeyer flask.
- Add ~10 dry ice chunks to the evaporation dish and let sit for 10 min. You can measure the temperature of this mixture. It should measure ~ negative 40°C.
- Next, calculate how much Ti(I-pro)4 you must add to your phage in order to achieve a 15:1 TiO2:phage ratio. The 15:1 gram ratio is based off of a calculation of the phage surface area to the surface area of the phage covered in TiO2, as the goal is to cover the phage completely with TiO2.
- Quantities you need to know:
- MW of phage = 1.8 x 10^7 g/mol
- MW of Ti(I-pro)4 = 284.22 g/mol
- MW of TiO2 = 80 g/mol
- Concentration of Ti(I-pro)4 = 0.96 g/mL
Think about the following progression: phage particle # --> moles phage --> weight phage --> weight TiO2 --> moles TiO2 = moles Ti(I-pro)4 --> volume of Ti(I-pro)4 to add
- Put on lab coat, gloves, and safety glasses for this next step. Add the calculated amount of Ti(I-pro)4 to the supercooled EtOH. Stir at least 5 minutes.
- Add your complexed phage solution to the EtOH:titanium and stir vigorously for 20 minutes.
- Remove the Erlenmeyer flask from the bath and place it back on the stir plate. Leave the mixture on the plate until it reaches room temperature. After a maximum of an hour your solution should turn grey (SWNT complexed phage) or white (gold complexed phage) indicating that the reaction has completed.
Part 2: Prepare a grid for TEM
Each group should prepare a TEM grid (so there might be 3 grids for each reaction of phage:SWNTs:TiO2). This will allow for some duplicates to be visualized in case the grid is damaged or different kinds of EM are being performed.

- Transfer the reaction to a 50 ml falcon tube. You can harvest the last few drops but not the stir bar using a larger magnet held to the bottom of the inverted jar (see image)
- Vortex the nanowires for 1 minute and immediately remove 5 ul of the nanowire suspension to place on the TEM grid that you have balanced in the specialized tweezers. HINT 1: The grid is "sided" and you want the shiny edge side up. If you are uncertain as to which side has the shiny edge, try looking under the dissecting microscope (12X magnification) to find the numeral "1" on the correct side. HINT 2: Treat the grid with care and use the tweezers only on the edge to minimize damaging the delicate mesh.

TEM grid balanced in tweezers - Allow the nanowires to settle onto the grid undisturbed for 5'. The EtOH will evaporate during this time. You can wick away any residual EtOH by touching the very edge of the grid with a Kimwipe.
- Wash the grid by adding 5 uL of 100% EtOH onto the grid. After 30 seconds you can wick away the EtOH.
- Wash the grid by adding 5 uL of sterile H2O onto the grid. After 30 seconds you can wick away the water and transfer the grid to a holder to visualize next time.
Part 3: Wash your phage:SWNTs:TiO2 nanocomposites
- Spin the remaining volume of nanocomposites in the clinical centrifuge at room temperature, 3000 rpm 10 minutes. At the end of this spin you should see dark material collected at the bottom of the tube. This is the material that will serve as the photoanode in our photovolatic device!
- Decant the supernatant into a chemical waste bottle in the chemical hood. Resuspend the nanocomposite material in 20 ml dH2O. Spin as before.
- Decant the supernatant into the sink.
- Hand the pellet of phage:SWNTs:TiO2 to the teaching faculty who will begin to prepare the paste needed to build them into the solar cell.
Part 4: Research pre-proposal
Pre-proposals will be due in lecture on M3D4 (11.29.12) at 11AM. That's coming up soon! Use your time today to look carefully at the requirements for this pre-proposal assignment. The requirements are posted here. Today you and your lab partner should work through the research topics you identified as interesting and decide on one (or at most two) that are worth researching more.
DONE!
ASSIGNMENT
Before M2D4 in lecture, every team must document a research pre-proposal by making and printing out a wiki page using the template found here.
Your pre-proposal will be evaluated based on its:
- creativity and innovation with its context
- clarity of goals and objectives
- clarity of writing and figure
- clarity of what results will achieve over current situation
- format
The reader of your pre-proposal should leave with the feeling of "Great idea! I wish I'd thought of that..."
Reagents list
- 100% EtOH
- Ti(I-pro)4 msds