20.109(F09): Mod 3 Day 5 Battery testing
Battery Testing
Introduction
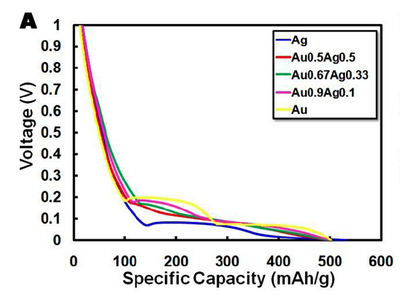
The theoretical capacity of a battery is the quantity of electricity involved for the electro-chemical reaction within the battery. For our gold nanowire batteries, the theoretical capacity is 510 mA*h/g, a value based on the 15:4 Li:Au ratio and the phase diagram for a lithium-gold alloy. Thus, we can calculate the "loading factor" for the Galvanostat to be 51 mA/g (theoretical capacity divided by the time to charge/discharge). This tells us how much current to use for a 10 hour discharge. Similarly, it's also possible to calculate the needed current to fully charge or discharge your battery. The mass of every electrode will be a little different, so the charge/discharge rates will be slightly different. But as an example, if one of the batteries has an electrode with precisely 1 mg of active material, then in order to discharge it in ten hours we'd have to apply a negative current of -51 uA.
Protocol
The electrodes that were prepared during last session were placed in a vacuum oven and dried at 100°C for four hours. They were then moved into a glove box and crimped into a battery, with the gold nanowires serving as the batteries cathode. Today instruction will be given on how to measure the charge/discharge on the Galvanostat.
