13 December 2008
From OpenWetWare
Jump to navigationJump to search
Date: 13 December 2008; Time: 0915; Agenda: Biofilm formation using M9 and LB Broth.
- 5ml of the 10ml of LB Broth culture (prepared on 12 December) was added to a flask containing 150ml LB broth.
- This flask was incubated for 5 hours (as determined by Mid-log phase earlier) in a 37 degrees shaking incubator at 250rpm.
- After 5 hours, we check the absorbance of the LB Broth culture:
- We first placed 3ml of pure LB Broth in a cuvette using a pipette in the fume hood. This serves as the 'blank' sample. The cuvette is then inserted in the spectrophotometer and the absorbance obtained was tared.
- The LB Broth culture was removed from the 37 degrees incubator.
- 3ml of LB Broth culture was removed from the flask and placed in a clean cuvette using a pipette. Cuvettes may be reused by rinsing with distilled water and then rinsing with the appropriate broth. The absorbance of this LB Broth culture was then read and its value recorded.
- Dilutions (on the original 3ml sample by adding more LB Broth) were done such that we obtain a final absorbance reading of 0.257 as this corresponds to the 1 McFarland Standard that we will use as our starting cell concentration. The results are attached as follows:
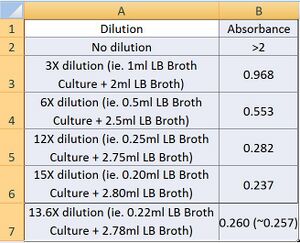
- As can be seen from the results, a 13.6X dilution was required to obtain 1 McFarland Standard.
- We took 0.1ml of the 13.6X diluted sample and proceeded to do serial dilution (100X per dilution; ie. 0.1ml of the 13.6X diluted sample + 9.9ml LB Broth per tube) and plated it on petri dishes (10^-8, 10^-10, 10^-12). The petri dishes were later incubated. This step is to verify that the 1 McFarland Standard (at 0.257 abs) is obtained, as literature states that the number of colonies obtained at this absorbance is 3*10^8.
- Also, 1ml of the 13.6X diluted sample is removed using a pipette in the fume hood and this is diluted with 29ml of LB Broth.
- Part of this 30ml mixture is poured onto the lid of a petri dish (just enough to cover the entire surface. This is done so that the multi-channel pipette (next step) is able to fit within the dimensions of the lid and obtain the required amount of mixture)
- A multi-channel pipette (8 pipettes) was used to obtain 0.23ml of mixture in each pipette tip. This was done in the fume hood.
- 0.23ml was obtained per pipette tip because protocol states that 22ml of the 30ml mixture is to be distributed in the 96 wells of the microtiter plate. Hence, by simple division, each well should contain about 0.23ml of mixture.
- As the 96 well microtiter plate has dimensions of 12 by 8, we decided to divide the plate equally into 2 halves: 6 by 8 for LB Broth culture and another 6 by 8 for M9 culture.
- Columns 1-6 will contain M9 broth culture and columns 7-12 will contain LB Broth culture.
- Columns 7-12 were accordingly filled with 0.23ml of LB Broth per well using the multi-channel pipette.
- For M9 culture, we obtained a total of 2ml of LB Broth culture and distributed it evenly into 4 centrifuge tubes (ie. 0.5ml per tube).
- The 4 tubes were centrifuged at 3000g for 10-15 minutes.
- In each tube, the supernatent was discarded and cells below were mixed with 0.5ml M9 prepared on 12 December.
- Mixing was done by pipetting up and down to ensure homogenity.
- Again, the absorbance of pure M9 was obtained and tared using the spectrophotometer.
- Subsequent absorbance values of diluted samples were obtained until the absorbance reached 0.257. See above for protocol. The results are as follows:
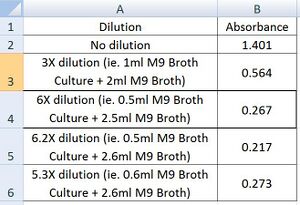
- As can be seen, an approximate dilution of 6X to obtain an absorbance of 0.267(close to 0.257) is required.
- Further dilution was again carried out (ie. 1:29; refer to procedures carried out for LB Broth medium) and finally the M9 culture was placed in the wells of columns 1-6 of the 96 well plate.
- The lid of the 96 well plate was replaced and the plate was incubated for 48 hours at 37 degrees and at 150 rpm.
- This marks the end of the biofilm growth process.