User:Norman Wang/DNA Isolation Via Agarose Gel Two Well Extraction
From OpenWetWare
Jump to navigationJump to search
- One step extraction of DNA from agarose gel electrophoresis without Gel Cutting & Purification. Similar to Invitrogen Clonewell protocol.
Methods
Materials
- 1.2% Agarose Gel with SYBR Safe (glows in both Blue Light and UV)
- UPA + 2 Log Ladder Mixture
- use Blue light 470nm LED illumination while extracting band (so it does not damage DNA)
- use UV illumination (more clearly visible) post extraction to verify
Protocol
- Cast gel using wide combs (x6 lanes), and line up the two sets of combs. First set comb wells for loading DNA, second set comb wells for extraction.
- Load enough buffer to slightly submerge agarose gel, leave thin layer of top surface un-submerged (so DNA doesn't run out of second row of wells)
- Prefill first set of wells with Nanopure H2O or buffer (if it has not already been accidentally flooded)
- Prefill second set of wells with Nanopure H2O
- Run until desired band gets close to second set of wells, use blue light so we don't damage DNA.
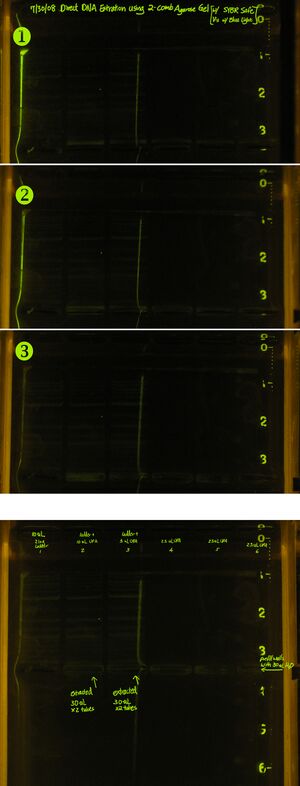
- Closely monitor band moving into extraction wells (second row of wells), use camera long shutter photograph to verify if band is too faint to see. Use flashlight to assist locating the well (flash on/off to see extraction well location/band fluorescence).
- Stop electrophoresis when band migrates into H2O filled well.
- Pickup DNA from second well using pipetter.
- After complete pickup, continue running the gel so band (if not picked up) continues to migrate.
- Later verify via photo (under UV or Blue Light illumination) that "some" if not all of the DNA band has been picked-up.
Results
- DNA was successfully picked up via this method, yield is low. Need more practice.
- Would help if I load more DNA next time!

Discussion
Future Improvements
- Load more than just 10uL of DNA (from PCR or Restriction Digest)
- Cast gel with:
- Thin loading (upper) well comb (for clearly defined running band)
- done, works ok.
- Fat pickup (lower) well comb (so it does not run back into gel immediately)
- done, work ok.
Add high salt cushion (3M Sodium Acetate + 0.1% Bromophenol Blue) to slow down charged moleculessalt traps DNA in the gel just before it comes out as liquid (agarose absorbs salt?), I tried 10x TAE and DNA become stuck in gel.
- Thin loading (upper) well comb (for clearly defined running band)
- Need brighter blue light illumination?
Conclusion
- This method can be used to extract DNA but has several major disadvantages:
- time watching for band to move into 2nd well is WAY TOO LONG (faster if cut DNA out as gel slice)
- unreliable amount of DNA extracted, I do not have time to perfect this method and get a quantitative comparison against faster methods such as gel slice cutting + Gel extraction kit or DNA_Isolation_Via_Agarose_Gel_Filter%2BMembrane_Extraction
- Things Learned
- SYBR Safe + blue light does NOT brightly stain DNA
- SYBR Safe stain would be great to use in conjunction with gel cutting method when DNA needs to be visualized and not damaged by UV while cutting
- Waiting for band to run into second well is a waste of time, use DNA_Isolation_Via_Agarose_Gel_Filter%2BMembrane_Extraction instead
- Other Agarose Gel DNA Band Extraction Methods
- Gel Extraction Kit (easy, but cost $$$)
- DNA_Isolation_Via_Agarose_Gel_Filter%2BMembrane_Extraction first try yielded 25%-40%, not bad... gives cleaner DNA than the Gel Extraction Kit (without the contaminant second slope on left when quantifying using spectrophotometer according to David Thompson of Liu Lab)
- Electroelution with home made device (need time to build a good electroelution apparatus, possibly design and manufacture it using 3D printer)