User:Mattia L. Avery/Notebook/Biology 210 at AU
Zebra Fish Embryology Lab
March 24, 2014
Introduction:
In the Zebra Fish Embryology Lab, the purpose was to study the effects of different concentrations of retinoic acid- vitamin A- on zebra fish embryotic development. Zebra fish embryos are ideal organisms to study the effects of retinoic acid because zebra fish are very sensitive to their environment and their development will be affected by abiotic factors such as temperature and light exposure. Several papers attested to the fact that retinoic acids cause zebra fish to develop deformities- particularly with structure and eye deformations- or even die prematurely. Throughout the study, irregularities were found in samples that contained doses of retinoic acid and there were significantly higher mortality rates in petri dishes that contained concentrations of retinoic acids.
Procedure:
1. A petri dish was filled with 20 mls. of Deepark water and then 20 zebra fish were transferred into the dish using a pipet.
2. Step 1 was repeated with a Petri dish with a diluted concentration of 10% retinoic acid solution and then with an undiluted concentration of retinoic acid.
3. The fish were observed throughout a two week period in increments, with results being recorded in the tables.
- On Day 8: 6 representative zebra fish were taken from the control and retinoic Petri dishes and observed in a depression slide using a 4x objective. These fish were then preserved in a small tube with a drop of tricaine solution per ml of water. Paraformaldehyde was also added to the tubes.
- On Day 15: The 4 fish (8 total) that were preserved in the paraformaldehyde solution were observed.
4. Dead embryos were removed periodically throughout the viewing period so that the petri dishes would not be contaminated.
5. When the embryos were 4 days old in the control (water) petri dish, 10 mls of water was removed and 25mls of fresh water was replaced. The same procedure was conducted with the retinoic acid Petri dish (except the added solution contained the diluted or undiluted retinoic acid.
6.Step 5 was to maintain a fresh aquatic environment upon every viewing.
Data
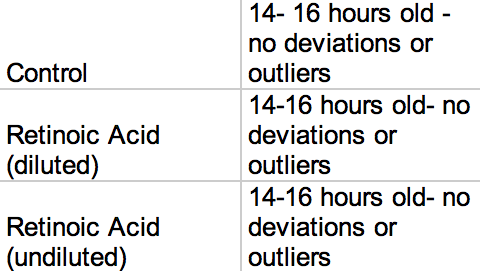
Table 1: Starting Ages
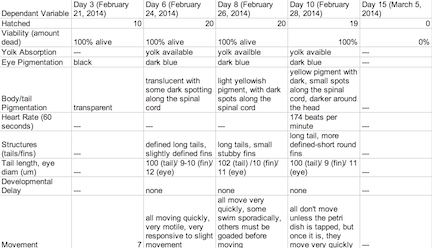
Table 1: Control Group
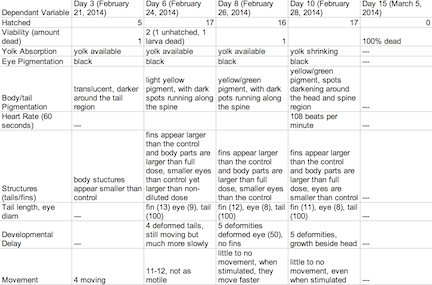
Table 2: Retinoic Acid Diluted Concentration Group
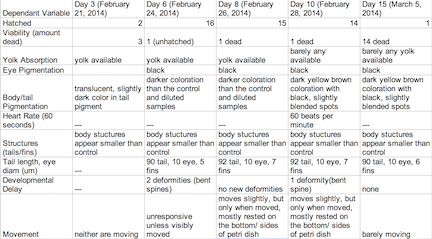
Table3: Retinoic Acid Undiluted Concentration Group
Lab 6
Conclusion
The objective of this experiment was to see how the retinoic acid would affect zebra fish embryo development. By comparing the results with those found in other experiments that conducted similar experiments with retinoic acid, we were able to provide follow up supporting many of the conclusions we found in these research papers. Retinoic acid affected eye structure, tail and spinal structure, movement, size and even body pigment. The zebra fish from the control group had no deaths whereas the diluted concentration had a few deaths, but the most deformities and the non-diluted concentration had the highest deaths, yet not as many deformities as the diluted concentration. In the future, we can use this knowledge of retinoic acid effects and the sensitivity of zebra fish embryos in experiments to apply them to future studies.
March 11, 2013
Introduction:
Many animals reproduce sexually and have gametes that fuse and form a single celled zygote. Through this lab, a better understand about how cell division and specialization occurred so that eventually a fully functioning new organism could develop. During the Embryology and Development Lab, the purpose lab explored embryology, and the identification of different stages of embryonic development and their comparison between organisms was possible due to the observation of three different species; frog, starfish and chick.
Materials:
Procedure 1: The first step was to observe a starfish egg.
1) The instructor projected a prepared slide with the stages of starfish development. 2) Stages were observed and noted. 3) Starfish zygote and unfertilized starfish egg were compared to one another.
Procedure 2: The second step was to examine frog development
1) Observe prepared slides of early and late cleavage of frogs beneath a microscope.
2) Observe live tadpoles.
Procedure 3: The third step was to observe chick development
1) In a dish, 72 hour chick embryos were placed beneath a dissecting scope and observed
2) Fertilized egg parts were identified using key indexes and observations
Data:
After viewing three samples beneath a microscope, we were able to make several conclusions, based on the observations taken, as seen in the figures below:
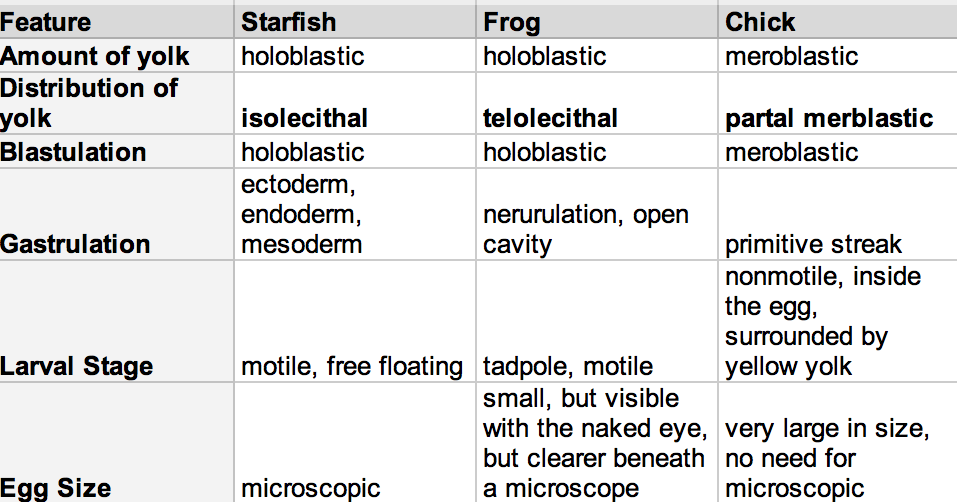 Figure 1: Comparing Embryo Aspects of a Sea Star, Frog, and Chick
Figure 1: Comparing Embryo Aspects of a Sea Star, Frog, and Chick
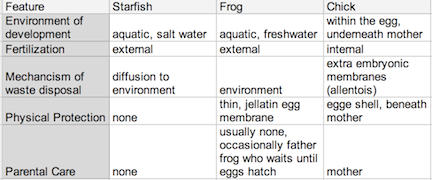 Figure 2: Comparing Environmental Aspects in a Sea Star, Frog, and Chick Development
Figure 2: Comparing Environmental Aspects in a Sea Star, Frog, and Chick Development
Conclusion:
The purpose of Lab 6 was to gain a better understanding of embryonic development using a starfish unfertilized and fertilized egg, a frog zygote and tadpole and a 72 hour chick embryo. By identifying embryological features and how different ecological development correlates to environment and other features, we were able to better understand the upcoming zebrafish lab project, and how to set up the experiment. The experiment studies how an environmental effect- our independent variable- effects our zebrafish embryonic development and functioning in life- our dependent variable.
Lab 5
February 28, 2014
Lab 5: Invertebrates in Transect #3’s Soil
In Lab 4, a Berlese Funnel was made by using the leaf litter sample collected. The Berlese Funnel forces small animals from the leaf litter into a tube of ethanol. By studying these tiny invertebrates, identification will provide more information about the conditions the ecosystem provides for certain species and where these species fit into certain niches within the transect. By identifying these species, an understanding about how structure- be it external or internal, affects the function of the species- which can be seen in the different types of mobility. Invertebrates from Transect 3 were identified in underneath a microscope and the diversity of the Transect was better understood.
Procedure:
Sample Invertebrates:
Observing the sample invertebrates provided during the lab was an exercise to prepare us for observing the invertebrates from the Berlese Funnel. Observation of invertebrates were found in this order: 1) Observe planaria beneath microscope 2) Observes nematodes beneath microscope 3) Observe annelids beneath microscope 4) Make notes about each After making careful observations about the three invertebrates from above, invertebrates from the Berlese Funnel were observed: 1) Disassemble the Berlese Funnel 2) Transfer preservative solution into a petri dish 3) Observe solution under microscope 4) Three organisms were noted and observed 5) Identify invertebrates using the dichotomous keys, the record results on table After observing invertebrates on the transect, potential vertebrates that could be found on the transect were also identified and recorded.
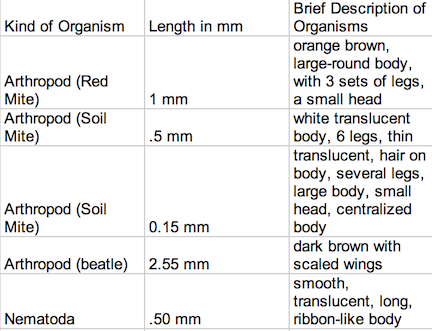
Data Despite the fact that there was some similarities in these worm-like invertebrates, they each had slightly different body types that allowed for different modes of transportation and function. The planaria were aided by cilia, and moved by stretching their bodies, gliding forward like a slinky toy. The nematodes moved their bodies like that of a shark, from side to side. And the Annelida moved contracted their bodies so that the front part would grip the bottom, to pull its elongated body forward. The lower part of its body would be dragged forward, like a wave in a crowd. The table shown in Image 1 identifies and describes the invertebrates found within Transect #3:
Transect Invertebrates
Image 1 beetle
Most of the organisms were nearly microscopic, and could barely be seen with the naked eye, and ranged in size from .15 mm to 2.55 mm. The largest organism found was a tiny, dark brown beetle and the smallest insect found was a soil mite. It appears that arthropods were the most common organisms taken from the sample
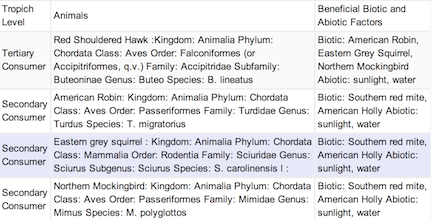
Transect Vertebrates
Image2
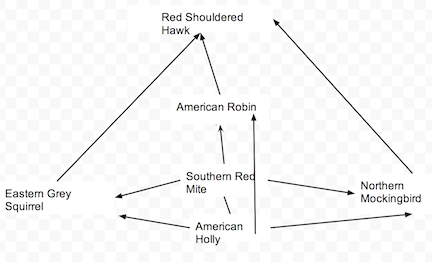
Transect Food Chain
Image 3
Conclusion: Through Lab #5, we were able to explore and discover the types of invertebrates within Transet #3 and by doing that, we were able to speculate on what vertebrates lived on the transect and how each respective creature fit into their niche. During Lab #5, we were able to understand how the structure of certain invertebrates, like the planaria, affected their function and their niche. Though we were unable to find many invertebrates or vertebrates, due to the weather and other testing factors, some data remains uncertain and based of remains and hypotheses. Even so, I feel we are one step closer to understanding our transect on a more biological level and as an ecosystem.
Lab 4: Observing and Identifying Vegetation of Transect #3 Tall Bushes
February 27th 2013
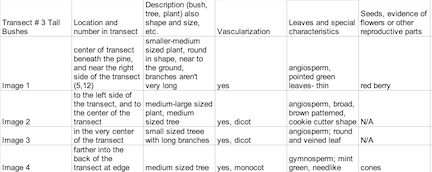
Introduction Plants vary in attributes (ex. vascular vs. nonvascular) to maximize their survival within their environment and are very versatile in types to fill many specific niches. Understanding how plants evolved to gain these characteristics can be imposed on the plants in Transect #3. There are three main types of plants: the Bryophytes, which are non vascular plants such as mosses, Tracheophytes, vascular plants with seeds, and Angiosperms, flowering plants. During lab, observing the characteristics of a Bryophyte moss called Mnium and an angiosperm, Lilium provided examples of the basic structures and aid in the identification of characteristics for five plant samples from the transect. The seven samples will be examined in terms of three general features: 1. Vascularization 2. Specialized Structures and 3. Reproduction.
Procedure Collecting/Observing plant samples from Transect #3: A sample of vegetation- leaves, leaf litter- was collected from Transect #3 and classified in the lab: 1) Dig into a place in the transect (location A on the transect map). Fill a plastic bag with 500g of soil and the rest with various foliage. 2) Take samples of five plants: keep in mind to look for a wide range of plants. Place in plastic bag. 3) In the lab, classify the various plants and record physical observations Observing fungi: In this lab, notes were also taken about fungi. Fungi are the primary decomposers of most ecosystems and therefore were necessary to study: 1) Observe the black bread-mold under the microscope
To begin Lab 4 to explore animals, an observation of the invertebrates that reside in our transects will be done. Since many animals are in hibernated for, the Berlese Funnel method must be used. 1) Pour 25 ml of 50:50 ethanol/water solution into the bottle 2) Fit screening material and tape to the sides of the funnel 3) Place funnel into the neck of the bottle 4) Carefully put leaf litter sample in the top of the funnel. Place 40 watt lamp above the funnel with the incandescent bulb about 1-2 inches from the top of the leaf litter. Cover with foil. 5) Leave the lighted setup on the lab bench for a week
Data
The leaves from Tall Bushes Transect were very slim, bright green and pointed. They are clustered in a radial pattern around red berries, which group in threes. Some were not typical leaves, but evergreen needles to conserve water during the wintertime. Most of the leaves found that were from Transect #3 were in the form of leaf litter. By the time they’d fallen to the forest floor, they had browned and were rotting. They were some differences such as much broader, larger in size and had a blunter in shape. They also had slim stems from where they were attached to a branch. The vascularization of the plants found at Transect #3 were vascular plants, some were gymnosperms while the fruiting plants were angiosperms. The longer, thinner leaves had vascular bundles that were scattered while the broader leaves had vascular bundles in a ring. Since it’s mid-winter, there was no evidence of flowers or spores and the berries from the holly could be neither identified as monocot or dicot since they are the shells that contain much smaller seeds, which were minute and black- too small to really observe. Fungi sporangia are one of the most important parts of the fungi because they enclose spores, which are necessary for asexual reproduction. Rhizopus stolonifer- black bread mold- contains stalks with filaments that are black and globule in appearance. This is a fungi because mycelium and sporangia in the structure, as seen in Image 6.
Image5
Image 1: American Holly Ilex opaca
Image 2: Red Osier Dogwood Cornus sericea
Image 3: Common Persimmon Diospyros virginiana
Image 4: Evergreen Coniferous Tree Thuja occidentalis
Image 6: Black Bread Mold
Image 7: PCR Reactions
Conclusion The purpose of this lab was to understand by observing the diverse characteristics of various plants within the transect. Learning about what characterizes fungi- which is the primary decomposer in Transect #3, also gave more insight to the ecosystems characteristics. Many species were identified and by doing this, the Transect #3 was better understood and gave insight to how the plants corresponded to their functions. By going through the motions of examining vascularization, specialization and reproduction in the plants within Transect #3, four plants found in the transect were identified and observed and to observe them, examinations of vascularization, specialization, and reproduction in the transect plants were made. Due to weather conditions, only four plants from the transect were found. Repeated studies should be done in the future to explore the transect more thoroughly during certain seasons, or even surrounding areas, which would further help examine the diversity of the ecosystem the transect represents. PCR reactions were received and information pertaining to the genetic makeup of the Transect #3 soil bacteria was found as well, giving more insight to Transect #3 A Berlese Funnel was made for next weeks lab.
Lab 3 Observing Bacteria in a Hay Infusion Culture
February 14, 2014
Introduction: This lab focused on the prokaryotes, which are grouped into the Domain Bacteria and the Domain Archaea, which includes mainly extremophiles- species that live in the most extreme environments. Bacteria are mostly unicellular and they are always prokaryotes. Bacteria have been classified by studying their morphology. The morphology of a bacteria species can be studied by looking at their cell shape, which comes in three basic forms: bacillus, coccus, and spirillum, and their staining characteristics. The most common stain is the Gram stain, which stains a bacteria either dark purple (Gram positive) or light pink (Gram negative) based on the structural characteristics of their cell wall. During this lab, the purpose of learning how to identify bacteria by comparing the colonies of bacteria found on the agar plates that contained the antibiotic tetracycline and the plates that did not. These agar plates were made from the serial dilutions of the Transect #3- Tall Bushes- Hay Infusion culture. By studying the bacteria found on Transect #3, a greater understanding about the nature of the bacteria found in the Tall Bushes ecosystem was established. Later, PCR Preparation for the Lab the coming week began.
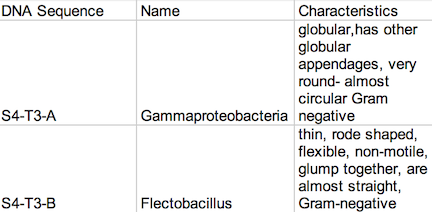
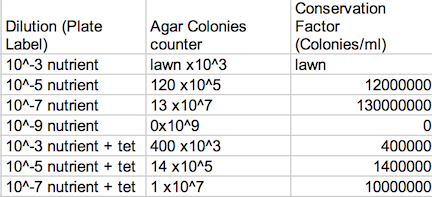
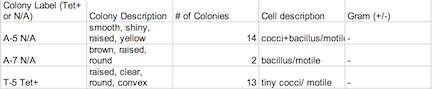
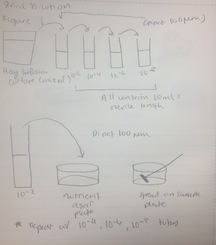
Procedure: 100-fold Serial Dilutions The first step before any bacteria was placed underneath the microscope was to observe the number of colonies on each nutrient agar plates. 1) Count the number of colonies observed on each plate. 2) Record the results (Table 1) Once the amount of colonies was counted and recorded, comparisons between the plates containing nutrient agar and then nutrient agar+tetracycline were made and the detailed observations were recorded (Table 2). Afterword, a wet mount of the bacteria was made. Two colonies from the nutrient agar plates and two from the tetracycline plates were taken. Wet mounts were made for each colony sample, and then these were observed under a microscope. Steps to making the wet mount were: 1. Scrape a piece of the bacteria colony from the surface of the agar plate and mix it with a drop of water on a slide 2. Place a cover slip on top of the slide 3. Observe the slide under the microscope and record observations 4. Repeat steps 1-3 with 2 other colonies (2 from nutrient agar; 1 from a tetracycline plate) After samples were observed under a microscope, the bacteria were prepared for Gram staining to see the colonies more clearly (by making them non-motile and stand out clearer due to the affects of staining) and to discover their cell wall components. 1. Scrape a piece of the bacteria colony from the surface of the agar plate and circle the area underneath the slide with a red wax pencil. 2. Bacteria side up, pass the slide through a flame three times 3. Cover the bacteria smear with crystal violet stain for 1 minute 4. Rinse off stain using water 5. Cover bacteria with iodine mordant stain for 1 minute 6. Rinse off stain suing water 7. Cover the bacteria smear with 95% and rinse 8. Cover with bacteria smear with safranin stain and rinse 9. Blot excess water on slide with a paper towel 10. Observe the gram stain slide under the microscope and record observations To prepare for the upcoming lab, PCR preparations were done directly after the current lab. Two bacteria colonies that showed the best characterization were taken from the nutrient agar plates. Their DNA was prepared to be isolated using these steps: 1. Transfer a colony of bacteria into 100µl of water in a sterile tube. 2. Incubate the tube at 100 °C for 10 minutes and then centrifuge 3. Put 5µl of supernatant in the PCR reaction
Data: Archaea could potentially survive on agar plates-since most of them are extremophiles, but I doubt there were many, if not any, archaea on our transect, since most archaea tend to grow in the most extreme environments. The Hay Infusion Culture had changed over the past week, such as the water had become a darker, muddier brown, and there was a thicker layer of dark brown debris on the bottom of the jar. The smell was less poignant because the Hay Infusion Culture was mainly evaporated after week, little water was left over as a result of the conditions. The Hay Infusion probably changed because the habitat shrunk due to evaporation, and more bacteria had to compete in a more competitive environment. The colonies on the tetracycline plates lacked lawn growth, which is typified in most bacterial growth. Bacterial colonies were much larger on the agar plates in comparison to the ones found on the tetracycline plates. For instance, nutrient agar plates with 10-5 dilution had 12,000,000 colonies per ml in comparison, a tetracycline plate with a 10-5 dilution had only 1,400,000 colonies per ml. For many of the tetracycline plates, as seen in Figure 1, bacteria failed to grow and only a couple fungi persisted. The plates with the tetracycline had significantly fewer colonies than the agar nutrient plates without tetracycline as shown on Table 1. This shows that Tetracycline stops bacteria from growing by interfering with their enzyme reactions. Therefore, the bacteria cannot reproduce and multiply. Tetracycline is very versatile in terms of what bacteria it can target; gram positive or negative, motile or non-motile, cocci or bacillus for example: Streptocouccus sspp and Neisseria gonorrheae.
Table 1: Agar Plate Colonies Assessment
Image 1
Table 2: Agar Plates Beneath a Microscope Assessment
Image 2
Conclusion: After successfully identifying and observing the colonies of bacteria on each plate, the discovery of how antibiotics, selection and the types of bacteria affected the survival of bacteria within the transect was made and more discoveries were made regarding the transects, even on a micro-biotic level.
Lab 2 Observing a Microsystem within the Hay Infusion Culture
February 8th 2014
Introduction The classification of organisms has been a long-standing debate amongst scientists. There are seven lineages that make up the Eukarya Domain and during the lab, examples of unicellular eukarya from the Hay Infusions culture are to be observed, studied, and identified to understand the characteristics of these organisms. To this, investigators must use a tool called “dichotomous key” to identify them. There are two very large groups of unicellular eukaryotes, the algae and protists. Using the Dichotomous keys, groups of organisms can be identified based on morphological choices. After making observations about size, shape, movement, and color of a single cell organism, an accurate organism will be identified correctly.
Procedure: 1) Make a wet mount of the sample with the known organisms and observe with the microscope. 2) Focus on organism/ characterize it/ measure the organism with the ocular micrometer. 3) Obtain a Key that describes eight known organisms. Use the key to determine the identity the organism. Confirm with the diagram. 4) Repeat with a second organism. Practice using the second key that has more organisms.
Procedure for Observation of Hay Infusion Culture:
In 500 mls of liquid, many species can be found. There are many niches. 1) Don’t disturb the culture while taking notes on description. 2) Take a few samples for microscopic observation, from two different niches, preferably. 3) Carefully place a small drop of liquid from the culture onto a microscope slide/place cover slip on top 4) Draw three organism from two niches and identify.
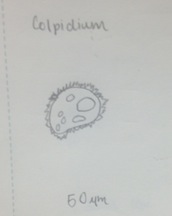
Figure 1 Classification: Colpidium Size: 50 µm Type: Protist, no photosynthesis
Data for Hay Infusion Culture The top of the Hay Infusion Culture had a thin layer of mold and smelled of wet earth. It was crème and coffee colored. Dark brown green shoots cling to the top along with creamy spots dotting the top. Organisms closer to the plant matter will gain from the effects of the photosynthesizing plant matter versus organisms farther away. Samples were taken from the leaf litter on the bottom, the thin layer of mold floating on top and near the shoots that cluttered around edges. Three different organisms found on the top of the culture:
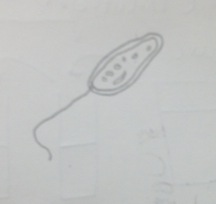
Figure 2 Paramecium pursaria: 100 µm, motile, protozoa
Figure 3 Colpidium: 50 µm, motile, algae, photosynthesizing
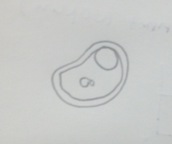
Figure 4 Peranema sp.: 20 µm, motile, protozoa Classification: Peranema Sp. Size: 20um Protist; no photosynthesis Observations: colorless, vibrating, flagella, moving quickly
Three different organisms found on the bottom of the culture:
Figure 5 Copidium: 50 µm, motile, algae, photosynthesizing
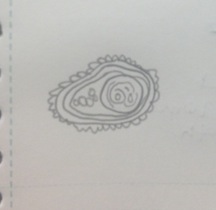
Figure 6 Paramecium aurelia: 130 µm, motile, protozoa
Figure 7 Paramecium pursaria: 100 µm, motile, protozoa
If the Hay Culture is left alone for two more months there is likely to be an increase of bacteria, due to rapid division with the help of nutrients like dry milk powder. There will also be an increase in protists because they would have more time to grow, and would eat the bacteria that had increased in numbers. But if the Hay Culture is not maintained overtime, the bacteria and protists will eventually die from lack of nutrients. These selective pressures, such as lack of nutrients and competition from space among species will also eventually limit the amount of species in the Hay Culture. Carrying capacity- number of individuals of a species that can survive in a particular niche, gives insight that with the increase in organisms, there will eventually be a rapid decrease due to selective pressures.
Conclusion: In the second lab, organisms were identified using the Dichotomous key. Species found in the Hay Infusion were discovered at separate niches, giving us insight to what organisms are found in certain niche locations. Nutrient agar and tertracycline plates were also successfully prepared and ready to be used in the upcoming lab. By observing the bacteria colonies for Lab 3 and the eukaryotes found in the Hay Infusion Culture this week, more details pertaining to our transect will be found on a microscopic level, giving us a larger impression of the ecosystem at large.
Procedure III Prokaryotes and some fungi from the Hay Infusion culture are to be observed for the following lab. Serial 100 fold dilutions of the culture are taken to inoculate on agar petri dishes. This is done to examine the bacteria.
Procedure
1) Label 4 tubes of 10mls of sterile broth with 2, 4, 6, and 8.
2) Label 4 tetracycline plates with 10-3, 10-5, 10-7, and 10-9. Repeat this with the nutrient agar plates.
3) Swirl the Hay Infusion Culture then take 100mls and add it to the 10mls tube labeled 2.
4) Swirl tube.
5) Take 100mls from tube 2 and add it to tube 4. Repeat this step with tubes 6 and 8.
6) Take 100mls from tube 2 (now 10-2) and place it on the 10-3 nutrient agar plate and spread it evenly.
7) Repeat step 5: tube 4 = agar plate 10-5, tube 6 = agar plate 10-7, tube 8 = agar plate 10-9
8) Repeat steps 5 and 6 with the tetracycline plates.
Figure 8
Observing Evolution Throughout the Volvocine Line
January 27, 2013
Lab 1 Part 1: Evolution is best studied by how traits change in a population overtime, while natural selection happens when certain traits are selected for or against due to environmental conditions. Chlamydomonas is the origin of the Volvocine line, an algae line that follows multi-cellular evolution. Evolutionary lines are related organisms that show a developmental progression of traits over millions of years, in this case, multi-cellularity. To test whether multicellularity became a favored trait among the Volvocine line, three samples were taken: Chlamydomonas, Gonium, Volvox, and placed under a high power microscope and then observed. Notes about the disparity between the living cultures were taken.
Procedure: In this experiment, we first observed three organisms that are part of the Volvocine line: Chlamydomonas, Gonium, and Volvox. This procedure included the following steps: 1. Prepare slides of each of the three organisms 2. Observe each of them under the microscope. Specifically observe the number of cells, colony size, functional specialization, and any reproductive specialization for each. Also observe patterns or trends in evolution. Record data in table (see below: Table 1).
Data The characteristics of each evolutionary specialization of members of the Volvocine line showed increasing complexity. Chlamydomonas were single celled organisms with a primitive form of sexual reproduction in which two vegetative cells functioned as isogametes. Performing asexual reproduction was another form of reproduction within chlamydomonas. Gonium was slightly more complex in that it formed colonies of up to 3-8 cells, as seen in the culture, and are held together by a gelatinous matrix. Volvox is the peak of evolutionary complexity. It is made up of thousands of cells, making a Volvox colony. Female gametes are larger than male gametes, making Volvox colonies as example of oogamy. Volvox also has indications of the beginnings of cell specialization.
Conclusion Though evolution does not always move towards increased complexity, the data shows that the Volvocine Line moved towards multicelluarity, which in this case was increased complexity. The data gives evidence to the fact that the Volvicine line evolved and adapted towards complexity, for example: Chlamydomonas- the least complex, being the origin of the Volvicine line, then Gonium the intermediate, and lastly Volvox, the most complexity.
Observing the Environmental Niches through Transect #3
January 30th, 2014 Lab 1 Part 2
Natural selection results in populations becoming more adapted and able to produce more offspring in their environment, therefore, species disparity will show at different transects, despite being within the same campus. In every ecosystem, there is balance and interactions that have shaped species. This was seen in the Volvicine line, which adapted towards multi-cellularity over time. By observing the transects, data allows us to learn about the aspects of our ecosystem and helps us observe how certain environmental pressures contribute to ecosystem evolution and diversity. Through further investigation and returning to the transect regularly, we’ll be able to observe the changes in Transect #3 throughout the semester, learn about other aspects of an ecosystem in our studies and observations of a transect. Specifically, in viewing the transect, our purpose is to observe how factors such as evolution have contributed to the diversity in the ecosystem. In the future, we will continue to observe our transect on a regular basis and further investigate how an ecosystem changes over a period of time.
Procedure Every group was assigned a different transect to discover how their transect affects the species within each transect. To study this: 1) Assigned Transect 3 2) Given 50 ml sterile conical tube to collect soil/surface plants 3) Located the parameters of Transect 3: 20ft by 20ft. 4) Using sterile conical tube, samples were collected from the soil and vegetation. 5) Studied the topography and drew a topographic map of the area 6) Described general characters and made note of the abiotic and biotic creatures within transect. 7) Take tube back to the lab. 8) With the sample from Transect #3, we then created a hay infusion culture. To create a hay infusion culture: 9) Weigh 10-12 grams of the sample and place in provided plastic jar with 500mls of deerpark water. 10) Add .1 grams of dried milk to the jar, place the top on the jar, and mix gently for 10 seconds 11) Remove top, label jar, and place the open jar in a safe place.
Notes taken during observation of transect 3: “Transect #3: Tall Bushes, January 27, 2013 The location is near the American University Amphitheater and was slightly removed from the center of campus, making it less disturbed by students. Transect #3 was interrupted by concrete sidewalks and the ground was littered with decaying leaves, stems and cigarette butts. The ground was shaded by stands of large shrubbery (Holly) and trees (Pine). The topography was slightly raised and hilly and in certain locations, there were lampposts. The abiotic factors were lampposts, cigarette butts, sidewalk, rocky soil, pebbles, and trashcan. The biotic factors were the weeds, brush, grass, saplings (maybe Oak or Maple-hard to tell without leaves) ivy, trees, and other ground foliage. More observations will be taken in the future.”
Conclusions and Future Plans:
- 3 Tall Bushes displayed a wide variety of organisms within the 20 by 20ft area near the Amphitheater and this diversity showed how natural selection and evolution creates a diverse range of interrelated species. We were successful in observing the aspects of an ecosystem, especially evolution. For the next labs to come, an observation of aspects of the transect, particularly through the hay infusion culture, will help us learn more about the ecosystem.
Very good start. Explained and described reason for each part of lab and gave some detail. Please enter newest entries at top of page moving older information downwards. SK