User:Kendall A. King/Notebook/Biology 210 at AU
Zebrafish Embryology Research Experiment: Dark Exposure
Purpose The purpose of this experiment was to document the effects that dark exposure has on the retinal development of Zebrafish Embryos. Data was collected over 13 days using a control group that undergoes normal daylight hours, and an experimental group that was exposed to total darkness. We hypothesized that the experimental group would develop slower and have less retinal development than that of the control group embryos.
Procedures
1. Begin by selecting 40 live Zebrafish eggs and divide them equally in to two groups of twenty and separate them in two petri dishes with 20 ml of Deerpark water in each petri dish. 2. Place the control group in a safe area in the lab room, and place the experimental group in a drawer with no light exposure. 3. Once eggs have hatched, select a representative one or two fish from both the control and experimental groups to observe under a microscope using the 10x objective lens. Record the heart rate by counting how many times the heart pulses in 15 seconds. Measure the length of the tale, the diameter of the eye, and record pigmentation and development of physical extremities. Document all data every two days and refill any Deerpark water that might have evaporated.
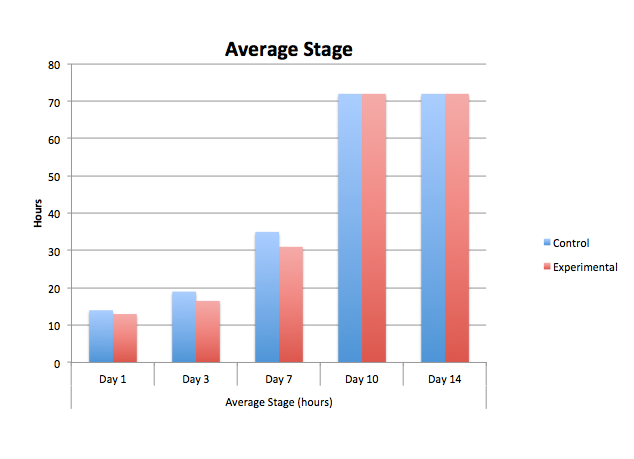
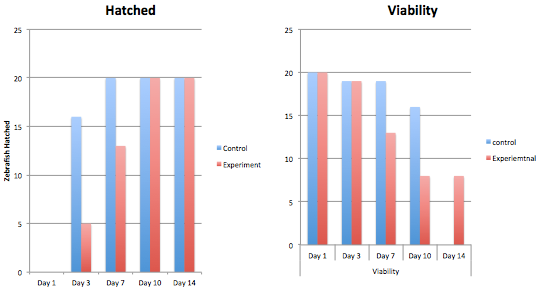
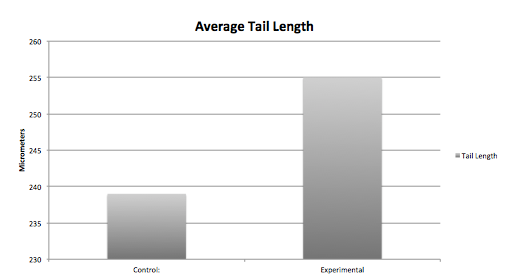
The graphs above contain the data collected over the 13 days in which the experiment was conducted. [Note: Day 13, all of the control group was found dead due to an inconsistency and harsh climate change]
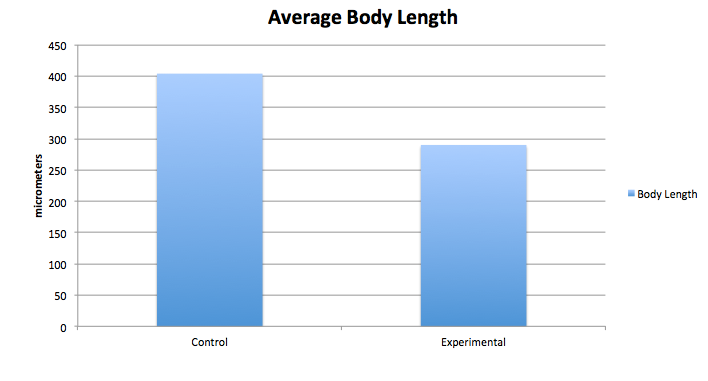
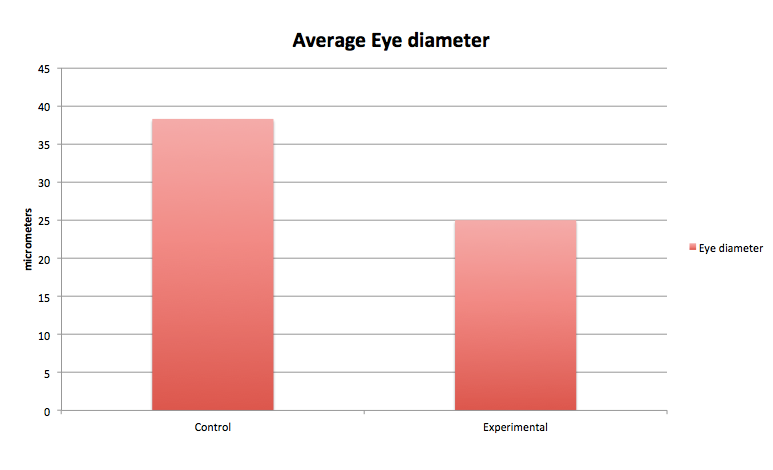
Conclusion From our data, we concluded that dark exposure prolongs the developmental process of the embryos' retina. The greatest differences between the control and the experimental group were the length of the body and tail, and the diameter and development of the eyes. Our hypothesis was supported by the data above.
testing
2/26/2014
Plantae and Fungi
As humans, we owe a lot to the world of plants and fungi. Scientists generally agree that all of the plants we know today evolved from aquatic green algae. The earliest plants were probably a type of moss, which are simple and non-vascular, belonging to the phyla of Bryophytes. After mass colonies of Bryophytes inhabited the oceans, and competition grew stiff, early Broophytes began to adapt to life on land. The land was virtually unoccupied, leaving plenty of space and resources for plants; however, life on land required a few adaptations that were not needed in the water: extreme fluctuation of temperature, access to water, access to nutrients and gasses, and reproduction by male and female gamete interaction. Thus Tracheophytes, further specialized and equipped with vascular systems, began the process of radiation, or the rapid diversion of new species from a common ancestor. This eventually lead to the evolution of angiosperms: the flowering plants that we depend so highly upon and use in domestic agriculture.
In this lab we observe the characteristics of Bryophyte moss and angiosperms, along with different species of plants from our designated transects on American University's campus.
Collecting plant samples: Procedures
1. Bring three bags to the transect. 2. Fill the first bag with leaf litter samples. Place about 500g (full bag) of litter into one bag. 3. Use other two bags to take representative samples from five different plants. 4. Find any seeds, pine cones, and other flowers and place in bag.
Collecting plant samples: Results
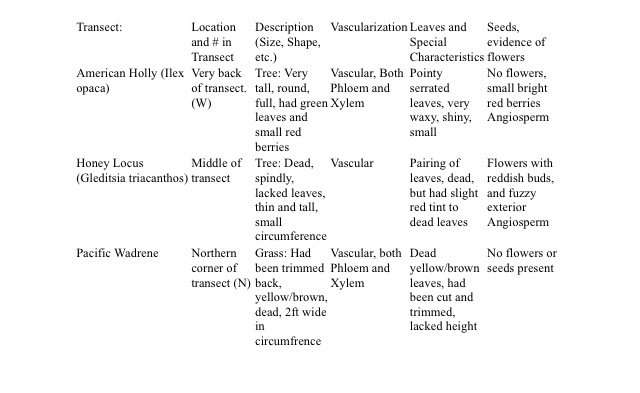
The above table classifies and describes the plant samples taken from the transect. (This includes the vascularization, leaf cluster and arrangement, and seed identification of each plant)
These are images of the collected samples. Each bag contains either a) Plant/twig material, b) seeds/flower material, or c) leaf litter material to set up our Berlese Funnels for Lab 5.
Dissection of plants and observation of Fungi:
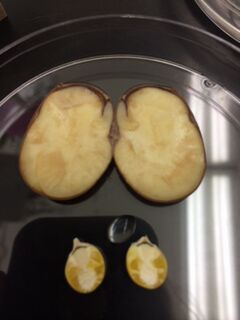
Images above display the dissection of different angiosperms. All organs of the lily were separated from each other and vascularization was observed. Also, seed coatings and nutrients were observed after the cross-section of both the kidney beans and kernels of corn.
Mold collected from previous labs were observed and photographed. This mold is a type of common fungi that grows and feeds off of decaying organic material. This mold included sporangia, the capsule containing individual spores, that are small, black bulbs at the tip of each mold strand. Most fungi produce sporangia that contain spores as a result of meiosis, which means the spores are genetically distinct and haploid. However, some fungi can produce spores through mitosis, resulting in genetically identical diploid spores.
Setting up Berles Funnel: Procedures
1. Pour about 25 ml of 50:50 ethanol/water solution into the small plastic tubes provided. 2. Cut, fit, and fix a piece of plastic screen, using tape and scissors, onto the inside neck of the large funnel. 3. Mount the funnel onto the stand and place the 25 ml water solution in it's tube directly below the mouth of the funnel. 4. Carefully pour the leaf litter from the 500g bag into the top opening of the funnel. 5. Cover the entire Berlese funnel with foil, and leave the light set up for one week on the lab tables.
Above are images of the Berlese Funnel that my group set up.
2/16/2014
Microbiology and Identifying Bacteria with DNA
Bacteria cover the face of the earth. They grow everywhere, including in our own organ systems, which can be both beneficial and harmful to our health. In this lab we observed and grew our own bacterial colonies on agar plates to document the different resistance or tolerance some species of bacteria may have against antibacterials. As we predicted, our colonies grew within the week, however, the presence of Archae species is unlikely because Archae are most commonly found in very extreme conditions. Our agar plates would hardly be considered extreme.
Hay Infusion Observation:
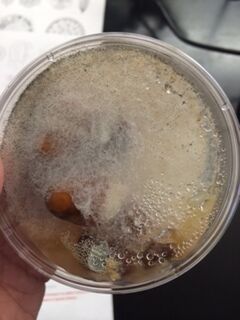
We observed our hay infusions one final time and documented the changes in smell and appearance over the duration of yet another week. The smell was much less potent, but had a subtle earthy and rancid smell, similar to that of carrion. The water lacked the same green-ness that it had the prior week. The water was clearer and the sediment was more compact at the base of the jar. Also, the filmy layer that once covered the surface of the water had dissipated. I believe that the change in smell is due to the lack of living organisms in the hay infusion. I believe the organisms have outgrown their small watery environment and probably used up all of the resources, leaving behind the smell of decaying matter. This also should affect the color of the water, in that the organisms have either floated to the top, have been consumed, or their empty shells have settled at the bottom, resulting in clearer water.
Procedures:
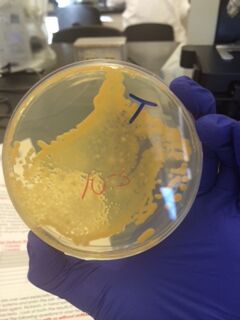
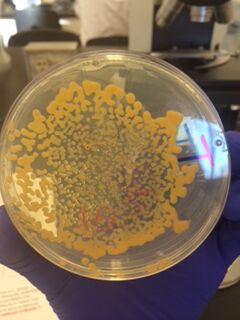
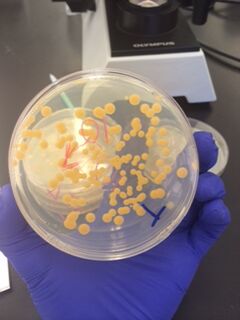
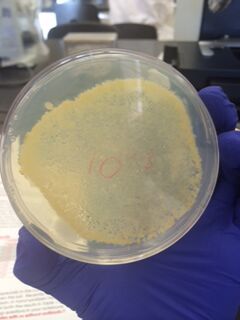
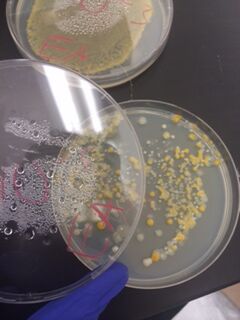
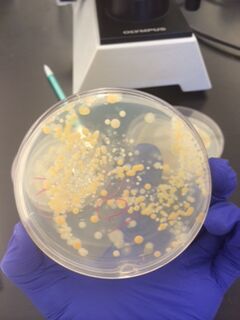
We began by observing all eight agar plates (five nutrient plates, and three antibacterial plates). Each plate consisted of different sized colonies, which were counted relatively. The number, size, and color of the colonies were then documented for each dilution.
Observations and Data:
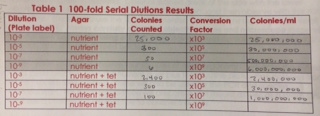
The following image displays a table depicting the calculated sizes of each dilution:
The size of the colonies are proportional to their percent dilution
The bacterial plates showed differences in color between the nutrient and antibacterial agar. The antibacterial agar plates were observed to have fewer variations in color, and only grew yellow bacterial colonies, while the nutrient agar plates grew a variety of colored bacteria including yellow, white, and clear. Both sets of agar growth varied in size and shape. The tetracycline had no noticeable affect on the number of bacterial growth, and resulted in only one or two similar species of bacteria that were resistant to the antibacterial solution.
Tetracycline was introduced to prevent the growth and spread of harmful bacteria in an effort to eliminate infections and illnesses. Tetracycline latches on to the bacteria's ribosomes, which then prevents the translation of RNA for further reproduction. However, some bacteria have overcome the tetracycline's technique and have grown resistant to it's effects on the RNA and DNA replication processes. This is a result of evolution through natural selection.
Procedures:
Four species of bacteria were observed under a microscope. Two of the samples were from our own agar plating, and the other two were prepared slides. The bacteria was observed under a x100 lens. The two sample from our own agar plates were then processed through graham staining. Each sample was doused in Crystal Violet and other chemicals and left to dry. Then the stainings were observed under a microscope and documented as Graham-positive or Graham-negative.
Next, using three samples of our nutrient and antibacterial agar plates, we ran PCR tests.
2/9/2014
Identifying Algae and Protists
Scientists have been using microscopes to identify and study microscopic organisms for many decades. There are many small organisms, both Eukaryotes and Prokaryotes, that may occupy just one droplet of water all while performing different roles within that specific ecosystem. Scientists call these individual roles niches. In our second Lab we observed and identified the microorganisms that were collected from our transect and processed through the previous lab's Hay Infusion.
Hay Infusion Observations:
Upon first glance, the water from out Hay Infusion was greenish in color and had a thin layer of slime on the surface and a layer of muck (plant material and dirt) on the bottom of the jar. Once opened, the jar reeked of a smell similar to that of bad breath.
Drops of water were then removed from varying depths of the jar and placed on a wet mount slide under the microscopes.
2/16/2014
Great job, Kendall! Some notes:
-Make sure you bold each entry date
-Make sure the top of the page has the 'newest 'entry first; so, move the 1/22/14 post down to the bottom; your future post for lab 2 should be the first thing I see when I open it next week
-Be sure to sign each entry at the end with your initials
-Make sure to upload photos for lab 1 by this Sunday
Awesome job!
AP
1/31/2014
Defining a Niche at American University
Objective:
Every organism has specific roles or functions in its environment that, collectively, can be called a niche. Groups of genetically-similar organisms form a species, and for every environment there are varying populations of each species that live among each other while competing for both biotic and abiotic resources. By studying a portion of land (transect) on American University's campus, the General Biology 210 class will observe how organisms and species interrelate in terms of ecology.
Procedures:
Transect Investigation Steps: Step 1. Identify each transect around campus, all of which are identified using four popsicle sticks placed at each corner of the 20'X20' of land. Step 2. Identify general characteristics of the transect Step 3. Identify the biotic and abiotic components of the transect. Step 4. Collect 50 mls of soil and ground vegetation as a sample using a sterile conical tube. Include all components representative of the soil and ground in this sample.
Hay Infusion Steps: Step 1. Weigh and separate 10-12 grams of the transect sample. Step 2. Place the 10-12 grams of the sample into a plastic jar containing 50 mls of deerpark water. Step 3. Add 0.1 grams of dried milk to the jar containing the water and transect sample. Mix gently for about 10 seconds with the lid of the jar on. Step 4. Label the jar with the names of the individuals in each group. Remove the lid from the jar and place both jar and lid in a safe place in the lab.
Data:
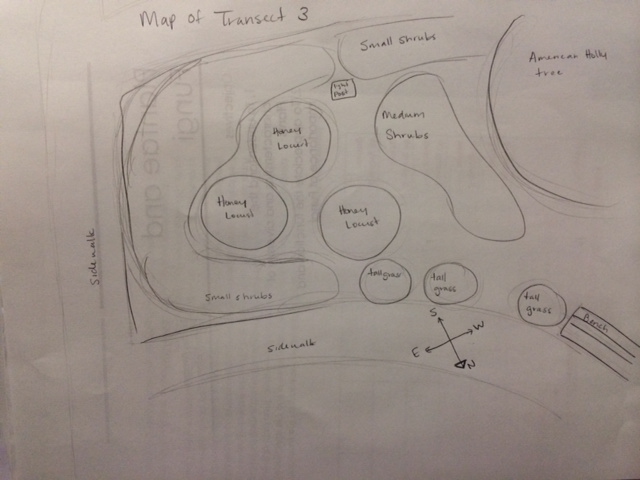
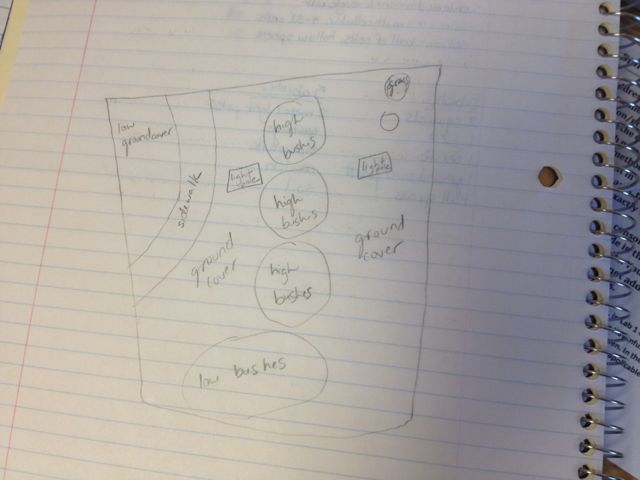
Transect Data: Location- The location of my group's transect is in an area of the botanical gardens behind the amphitheater and to the right of Bender Arena. Topography- The transect is between two sidewalks in a slightly-shaded area and relatively flat land. Abiotic Factors- Concrete, soil, street lamp, sunlight, plenty of open space, fresh air, some trash (an empty yogurt container and a plastic bottle), one bird's nest Biotic Factors- ground-coverage plants, small and medium sized shrubs, small trees, one larger holly tree, bark, tall grasses
Hay Infusion Data: Data for the hay infusion will be documented under future lab records.
Conclusion:
From our first lab assignment I have documented both biotic and abiotic factors, studied the topography and location of the transect, and collected samples of soil and ground vegetation. In the future my group will observe the microorganisms in the soil sample that was collected and added to the water/milk mixture. From these observations we will document the diversity of the organisms found in the soil from our transects and possibly experiment further with the different microbes and how they function in our transect.
--Kendall A. King 14:52, 31 January 2014 (EST)KAK
1/22/2014 Assigned lab notebook and entering text successfully --Kendall A. King 14:52, 31 January 2014 (EST)KAK