Tasks for September 10
- To complete film synthesis from September 5
- To begin film preparation of ionic liquid modified clays
- To analyse DSC data
Completion of Film Synthesis
Continued from Dr. Harting's procedure
- Placed in sodium sulfate solution, followed by 1% HCl solution and finally in Sodium Bicarbonate
Film preparation of ionic liquid modified clay
- Procedure followed as detailed by Dr. Harting
- Added 1.12 g of of Sodium Montmorillonite (NaMT) to a scintillation vial containing 20mL of 50:50 HPLC grade water:ethanol.
- Added 0.523 g of tributylhexadecylphosphonium bromide (THP Br) to exchange 100% of the Na from the clay.
- 1.12 g NaMt × (92×10-3/100g NaMT)×(507.65g THP Br/mol) = 0.523 g of THP Br
DSC (Differential Scanning Calorimetry) Data
Note: To calculate mass of water evaporated the following equation was used: q=mHv. So that mass of water=(q×mass of sample)/Hv where Hv=2.23×103 J/g<br.>
PVA film in 2 ppm MG solution<br.>
 <br.> <br.>
- Onset of water evaporation: 99.59ºC
- Heat of hydrate decomposition: 830.4 J/g
- Mass of water evaporated = 0.00144 g
- Major peaks in water:
- Minimum at 134.73ºC, other peaks at 101, 103 ºC
- Glass transition:
- Top curve inflection point: 68.58ºC
- Bottom curve inflection points: 69.39, 85.00ºC<br.>
PVA film in 8 ppm MG solution<br.>

- Onset of water evaporation: 100.91ºC
- Heat of hydrate decomposition: 1130 J/g
- Mass of water evaporated = 0.00177 g
- Major peaks in water:
- Minimum at 103.58ºC, other peaks at 107, 117 and 120ºC
- Glass transition:
- Top curve inflection point: 68.11ºC
- Bottom curve inflection points: 68.94, 87.66 ºC<br.>
PVA film in 80 ppm MG solution<br.>
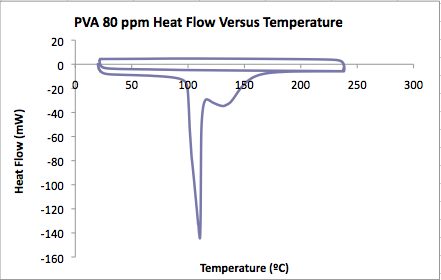
- Onset of water evaporation: 99.82ºC
- Heat of hydrate decomposition: 1458 J/g
- Mass of water evaporated = 0.00257 g
- Major peaks in water:
- Minimum at 110ºC, other peaks at 117 and 130.
- Glass transition:
- Top curve inflection point: 68.17ºC
- Bottom curve inflection point: 68.56ºC<br.>
PVA film in 200 ppm MG solution<br.>

- Onset of water evaporation: 99.77ºC
- Heat of hydrate decomposition: 938.2 J/g
- Mass of water evaporated = 0.00147 g
- Major peaks in water:
- Minimum at 125.54ºC, other peaks at 101, 105 and 136
- Glass transition:
- Top curve inflection point: 68.36ºC
- Bottom curve inflection point: 69.15 ºC<br.>
PVA-Clay film in 2 ppm MG solution<br.>

- Onset of water evaporation: 100.51ºC
- Heat of hydrate decomposition: 1276 J/g
- Mass of water evaporated = 0.00232 g
- Major peaks in water:
- Minimum at 107ºC, other peaks at 109, 111 and 125ºC
- Glass transition:
- Top curve inflection point: 68.16ºC
- Bottom curve inflection point: 68.70 ºC<br.>
PVA-Clay film in 8 ppm MG solution<br.>

- Onset of water evaporation: 98.55ºC
- Heat of hydrate decomposition: 865.9 J/g
- Mass of water evaporated = 0.00213 g
- Major peaks in water:
- Minimum at 101.19ºC, other peaks at 104, 130ºC
- Glass transition:
- Top curve inflection point: 68.59ºC
- Bottom curve inflection point: 69.02 ºC<br.>
PVA-Clay film in 80 ppm MG solution<br.>

- Onset of water evaporation: 100.76ºC
- Heat of hydrate decomposition: 1044 J/g
- Mass of water evaporated = 0.00158 g
- Major peaks in water:
- Minimum at 101.94ºC, other peaks at 105, 108ºC
- Glass transition:
- Top curve inflection point: 68.27ºC
- Bottom curve inflection point: 68.50 ºC<br.>
PVA-Clay film in 200 ppm MG solution<br.>
 <br.> <br.>
- Onset of water evaporation: 99.70ºC
- Heat of hydrate decomposition: 1138 J/g
- Mass of water evaporated = 0.00177 g
- Major peaks in water:
- Minimum at 108ºC, other peaks at 110, 131ºC
- Glass transition:
- Top curve inflection point: 68.54ºC
- Bottom curve inflection point: 67.97 ºC
|







