Macrophages and Their Interactions with Engineered Tissues, by Veronica Murray
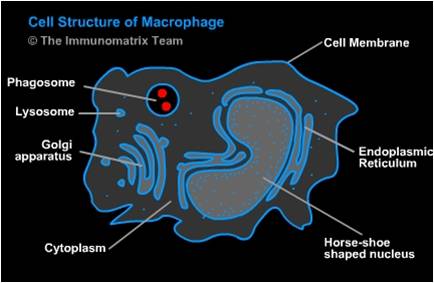
Background
When a tissue is damaged the immune system responds and monocytes are recruited to the tissue where they differentiate into macrophages. One of the difficulties with studying macrophages is that they exist in every tissue in the body but the type and functional phenotype vary based on location, situation, and injury. For example, osteoclasts are a type of macrophage. There is a wide variety of macrophage function but some common and important functions include "eating" apoptotic cells and pathogens and producing immune effector cells.
Why Study Macrophages?
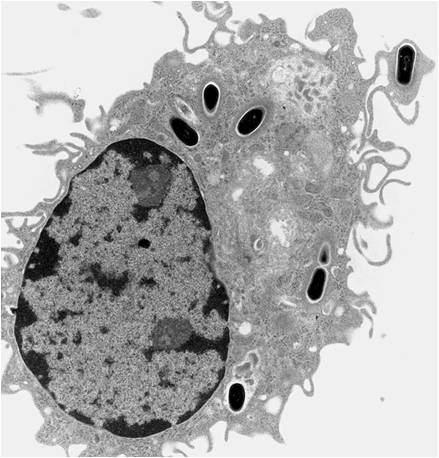
Biocompatibility is primarily determined by the host's response to the surface of the implanted material. Tissue location, material form, and material size all influence a person's reaction to an implanted material or engineered tissue. Immediately after the implantation, the surface of the material coated with proteins that direct cellular adhesion and activation. Some of these proteins recruit macrophages which then can recruit more macrophages. Over time, macrophages can cause stress cracking and severe damage to implants. If more is known about how macrophages function, materials can be designed to work with them instead of against. This greatly increases the lifetime of engineered tissues and thus of patients with implanted biomaterials.
Adhesion and Fusion
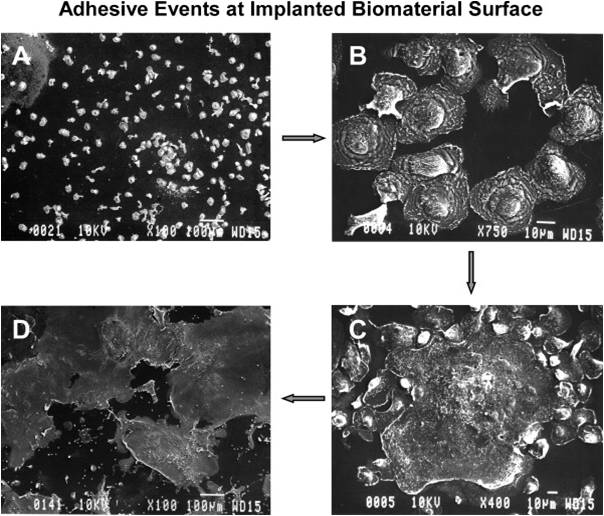
Many different mechanisms have been found for macrophages adhering to surface. Research pertaining to the specifics is current, ongoing, and the conclusions differ. Generally speaking, one of classes of signals that attract macrophages to materials are chemokines. Chemokines are simply cytokines that have chemoattractative properties. Chemoattractants are released by platelets soon after injury: transforming growth factor (TGF-β), platelet-derived growth factor (PDGF), CXCL4 (Platelet Factor, PF4), leukotriene (LTB4), interleukin (IL-1) specifically. Chemoattractants are aided by podosomes, specialized macrophage adhesion structures formed in the early stages of cell adhesion. Once macrophages have bound to the material surface, they can produce intracellular signals to modify/control other macrophage behavior. Production of tumor necrosis factor (TNF-α), IL-6, granulocyte-colony stimulating factor (G-CSF), and granulocyte macrophage colony stimulating factor (GM-CSF) can call more macrophages to the site.
Macrophages also express integrins, or proteins that function by attaching the cell cytoskeleton to the extracellular matrix. Three types of β chains (β1, β2, β3) are expressed and it has been found that three types of β2 integrins assist in recruiting and adhering macrophages to engineered tissues/biomaterials [1]. They are characterized as intercellular adhesion molecules (ICAMSs) and are denoted αL/β2, αM/β2, and αD/β2.
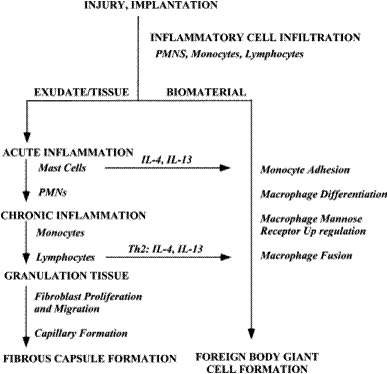
Additionally, after adhering to the surface macrophages’ cytoskeletons can remodel to allow for spreading over the surface of the material. In this manner, macrophages completely surround the material in a short period of time. Depending on material properties, this can be very harmful or fairy benign. The most harm tends to occur when macrophages detect large particles. One macrophage alone can only phagocytose very small particles (<5 μm). Larger particle sizes (>10 μm) induce the formation of foreign body giant cells (FBGCs).
Activation
Macrophages are functional without any additional activation and activation does not increase phagocytotic behavior. However, different types of activation do have other very important impacts on behavior and function. Classical activation allows macrophages to kill intracellular pathogens, upregulate pro-inflammatory cytokines, inhibit anti-inflammatory cytokines, and produce nitric oxide. These behaviors are all damaging to a patient when these actions are directed against their implant or engineered tissue [2]. To be classically activated, macrophages need two distinct signals. First, cytokine IFN-γ primes the macrophage for activation and then tumor necrosis factor (TNF) actually activates the macrophage.
A promising area of study is alternatively activated macrophages. Macrophages that are alternatively activated inhibit pro-inflammatory cytokines and promote anti-inflammatory cytokines. To be alternatively activated, macrophages need to interact with IL-4. When inflammation is inhibited, healing of injured tissues can progress. Over time macrophages can transition from classically activated to alternatively activated but the reasons and methods of this transition are debated.
Inflammation
The human inflammatory process is highly regulated and has many components. During inflammation, macrophages have three specific functions: antigen presentation, phagocytosis, and immunomodulation. This essentially results in a lot of control over the initiation, maintenance, and resolution of inflammation. During the process of inflammation macrophages are activated and deactivated. To reiterate material from above, the specifics have not been entirely agreed upon but it is known that activation signals are mainly cytokines and extracellular matrix proteins.
Acute inflammation is the inflammation that happens immediately after the implantation of engineered tissues and lasts less than a week. This type of inflammation is influenced by histamine-mediated phagocyte recruitment (where macrophages are a type of phagocyte). H1 and H2 histamine receptor antagonists can greatly reduce the recruitment of macrophages during this phase and thus reduce inflammation. Chronic inflammation occurs after this and lasts about two weeks. Chronic inflammation lasting longer than this indicates infection.
For repair of damaged tissues, the host needs inflammation to be inhibited by the removal or deactivation of mediators and inflammatory effector cells. The most prevalent deactivation signals are anti-inflammatory cytokines.
Effects of Different Materials
| Material | Common Use | Degradation | Prevention |
|---|---|---|---|
| Polyethylene | Artificial Joints | Surface Oxidation | Antioxidants added |
| Polypropylene | Suture Material | Surface Oxidation | Antioxidants added |
| Most Polyesters | Resorbable sutures | Complete degradation and resorbtion is desired | None |
| Polyethylene terephthalate (Dacron) | Vascular graft prostheses | Minimal | |
| Polyurethanes | Varies with specific chemistry |
[1]
Current Research
In this section two papers are summarized. They each represent a type of research happening in this field currently and their results suggest areas of future study.
Case Study 1
Basic Premise: In vitro human monocyte culture to determine how adherent monocyte/macrophage cytokine production are influenced by surface chemistry
Method: Polyethylene terephthalate (PET) surface modified to create hydrophobic, hydrophilic, anionic, cationic surfaces Isolated human monocytes cultured onto surfaces ~10 days with or without interleukin-4 (IL-4)
Results:
| Surface | IL-10 Expression | IL-8 Expression |
|---|---|---|
| Hydrophilic | Increased | Decreased |
| Hydrophobic | -- | -- |
| Cationic | Decreased | -- |
| Anionic | Increased | Decreased |
Conclusions: Hydrophilic and anionic surfaces inhibit adhesion and IL-4–mediated macrophage fusion into FBGCs Thus, hydrophilic and anionic surfaces promote anti-inflammatory response. [3]
Case Study 2
Basic Premise: Assess inflammatory response in patients with failed orthopedic implant
Method: Analyze macrophages and evaluate their possible role in erosive inflammatory lesions within the bone
Results: “A considerably greater percentage of RFD1 positive Mφ [macrophages] and FBGC was noted in the interfaces from cases with a high level of detectable metal particulate wear debris (mean 80%, range 60%–90%) than in cases with polyethylene wear debris (mean 30%, range 0%–50%), p 0.0001.” [5]
Conclusions: Macrophages degrade both metal and polyethylene Polyethylene degradation is 50% less than metal degradation
Summary/Review
Macrophages will adhere to engineered tissues implanted in the body. They can damage engineered tissues and biomaterials over time, causing irreperable damage. Fortunately, it is possible to control biomaterial adherent macrophages by altering surface/substrate chemistry. Successful future work will increase the lifetimes of engineered tissues and thus improve the lives of patients by reducing the number of implantations or surgeries required.
References
[1]Anderson, J. M.; A. R.; Chang, D. T. Foreign Body Reactions To Biomaterials. Innate and Adaptive Immune Responses in Tissue Engineering. 2008, 20, 86–100. http://www.sciencedirect.com/science/article/pii/s1044532307000966.
[2]Mosser, D. M. The Many Faces of Macrophage Activation. Journal of Leukocyte Biology. 2003,73, 209–212.
[3]Brodbeck, W. G.; Matsuda, T.; Colton, E.; Ziats, N. P.; Anderson, J. M.; Nakayama, Y. Biomaterial Surface Chemistry Dictates Adherent Monocyte/Macrophage Cytokine Expression In Vitro. 2002, 18, 311–319.
[4]Fujiwara, N.; Kobayashi, K. Macrophages In Inflammation. CDTIA Current Drug Target -Inflammation & Allergy. 2005, 4, 281–286. http://www.eurekaselect.com/90593/article.
[5]Al-Saffar, N.; Revell, P. A.; Kobayashi, A. Modulation Of the Phenotypic and Functional Properties of Phagocytic Macrophages by Wear Particles from Orthopaedic Implants. Journal of Materials Science: Materials in Medicine. 1997, 8, 641–648.
