IGEM:IMPERIAL/2009/cell encapsulation methods/crystal encapsulation
Crystal Encapsulation Tutorial This is only a brief intro, I've provided links to useful papers on biomineralization on the main encapsulation page.
http://openwetware.org/wiki/IGEM:IMPERIAL/2009/Brainstormings
My advice to you all is to try to read as much as possible as this is the best way to get to grips with the area.
Background:
A crystal is made of an orderly repeating pattern of molecules extending in all three spatial dimensions.
Salt solutions contain positively charged ions (cations) and negatively charged ions (anions). For instance, a calcium phosphate solution contains both calcium and phosphate ions.
Since cations carry a positive charge, they are attracted to chemical species that carry a negative charge. If a protein was to carry many negative charges, then cations would nucleate around it. The high density of cations (calcium ions) around the nucleation site attracts the anions (phosphate ions) in the solution initiating crystal growth.
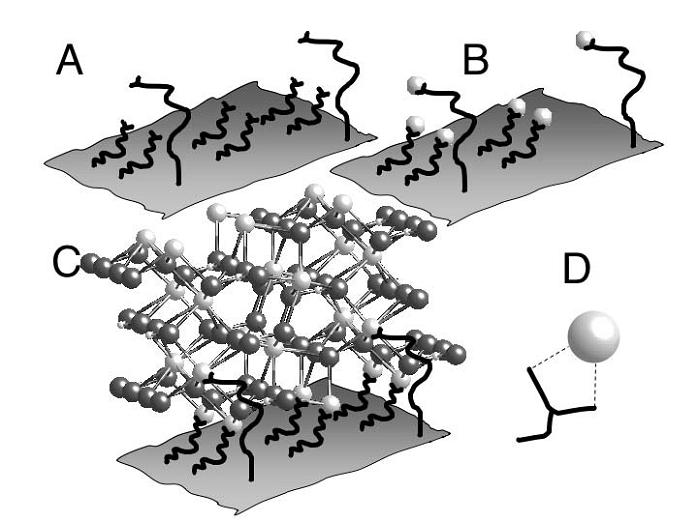
A = Proteins with negatively charged ends attached to a sheet. B = These proteins have nucleated site for positively charged cations (white balls). C = Growth of crystal lattice. D = Carboxylate residue associated with a cation.
Important points:
1) We can encapsulate cells in any crystal we like. Different crystals will have different functions.
2) We can vary crystal thickness by altering the time in which the cells spend in a high ionic strength solution.
Practical Approach to Cell Encapsulation: If we wish to encapsulate cells, we must first give the outside a net negative charge. This can be done via chemical treatment e.g. PAA (poly-acrylic) or by using the negatively charged amino acid glutamic acid.
The following paper demonstrates that a string of glutamic-acid residues is nearly as good as rat bone sialoprotein at inducing the deposition of calcium phosphate (hydroxyapatite). This is important as there has historically been much debate over whether deposition is due to either sialic acid or acidic amino acid residues.
Which chassis would suit encapsulation best?
Both E.coli & B.subtilis have a negatively charged cell wall. This means that they probably possess an innate ability to attract cations. If we were going for simple crystal encapsulation, I would recommend increasing the negativity of the cell wall by attaching multiple poly-glu sequences.
If you decide to do this, the following paper is a good intro:
http://www.ncbi.nlm.nih.gov/pubmed/12480350
If you were looking to control crystal deposition then Lactobacillus would be your best bet. As you know, Lactobacillus is covered in an S-layer. Unlike most bacteria, the S-layer of Lactobacillus is positively charged! It is likely that this is because Lactobacillus often lives in environments with high ionic concentrations (e.g. milk) and does not wish to become encapsulated. We can use this to our advantage in that it gives us a blank slate on which to attach our negatively charged poly-glu sequences.
If you decide that you which to attach poly-glu sequences to Lactobacillus, you must read the following papers (in order of importance):
http://aem.asm.org/cgi/content/abstract/68/12/5943
Heads Up S-Layer Display: The Power of Many
Participation of a Cyanobacterial S Layer in Fine-Grain Mineral Formation