IGEM:IMPERIAL/2009/Encapsulation/PKU
Phenylketonuria - PKU
Summary
<html>
<table border=1>
<tr>
<td><b>Solution</b></td>
<td><b>Enzyme(s) involved</b></td>
<td><b>Cofactor?</b></td>
<td><b>Viable in small intestine?</b></td>
<td><b>References</b></td>
<td><b>Feasible</b></td>
</tr>
<tr> <td>Phenylalanine hydroxylase. We would need to make the E. Coli secrete a modified PAH enzyme that is less susceptible to proteolysis.</td> <td></html>Phenylalanine Hydroxylase<html></td> <td></html>BH4<html>, this is what the only drug for phenylketonuria (Kuvan) targets. Kuvan is a functional analog of this co-factor thus only targeting a subset of PKU patients</td> <td>The enzyme can be rendered protease resistant via full phosphorylation and operates at pH similar to that of the small intestine.</html>[1]<html></td> <td></html>Paper<html></td> <td><b><font color="orange">Doable, not the easiest of solutions</font></b></td> </tr>
<tr> <td>Phenylalanine ammonia-lyase</td> <td></html>Phenylalanine ammonia-lyase<html></td> <td>None</td> <td>The enzyme was shielded against proteases either by secreting a protease inhibitor (aprotinin) or by retaining it into the transfected E. Coli cells. Problems with these two approaches are -respectively- that we would inhibit normal digestion of peptides with proteases and that we would need to keep the modified bacterium alive.</cite></td> <td></td> <td><b><font color="orange">Too many drawbacks</font></b></td> </tr> </table> </html>
Absorption in gut
Phenylalanine is absorbed as an amino acid in our gut. [2] This is because the hydrolysis of peptides involving phenylalanine is fast enough. It takes 175 minutes or approximately 3 hours for all phenylalanine related peptides to be digested, after which, phenylalanine will be present as an amino acid to be absorbed. [3] For this experiment, volunteers are fed a milk-protein meal.
 [4]
[4]
Metabolism of Phenylalanine
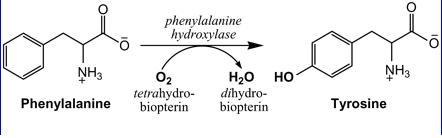
Phenylalanine is fully catabolised to CO2 and water, after its hydroxylation to tyrosine. This is the rate limiting step in the whole process. The complex system requires PAH, tetrahydrobiopterin co-enzyme (BH4) and several enzymes for the regeneration of BH4. The characteristics of phenylalanine metabolism have been modelled[5][6].
Conversion to tyrosine
Phenylalanine is converted to tyrosine by phenylalanine hydroxylase. Tyrosine synthesis in the body depends on the availability of phenylalanine. When phenylalanine intake is limited, tyrosine can become a conditionally indispensable amino acid.
The carboxyl carbon of the tyrosine is released as CO2 when tyrosine degrades. The remainder of the molecule is converted to fumarate or acetoacetate, which enter the tricarboxylic acid cycle.
BH4
PAH PhenylAlanine Hydroxylase
This is the enzyme that catalyses the reaction from phenylalanine to tyrosine. It is naturally produced by the liver. However, it was expressed in E. Coli and shown to be functional at pH 7.0[1]. Furthermore, phosphorylation by the C-subunit kinase rendered the enzyme resistant to the Xa protease (fig 1 below) which is a member of the serine protease family[1]. The serine protease family includes some of the most abundant proteases present in the human gastro-intestinal tract such as chemotrypsin-like, signal peptidases (More Info on Serine Proteases).
Therefore we think that by fully phosphorylating the PAH enzyme during its production process, we can protect it against the protease-rich environment of the small intestine.
The PAH enzyme is activated by L-Phenylalanine[1] and thus is expected to be activated in the environment of the small intestine where food is naturally degraded into amino acids. These amino acids are subsequently absorbed by the gut.

References
- Døskeland AP, Martinez A, Knappskog PM, and Flatmark T. Phosphorylation of recombinant human phenylalanine hydroxylase: effect on catalytic activity, substrate activation and protection against non-specific cleavage of the fusion protein by restriction protease. Biochem J. 1996 Jan 15;313 ( Pt 2)(Pt 2):409-14. DOI:10.1042/bj3130409 |
- Mendez R, Aswad S, Dessouki A, Cicciarelli J, and Mendez RG. Costs and financing of kidney transplantation in the United States. Transplant Proc. 1992 Oct;24(5):2127-8.
- Nixon SE and Mawer GE. The digestion and absorption of protein in man. 2. The form in which digested protein is absorbed. Br J Nutr. 1970 Mar;24(1):241-58. DOI:10.1079/bjn19700024 |
- Nixon SE and Mawer GE. The digestion and absorption of protein in man. 1. The site of absorption. Br J Nutr. 1970 Mar;24(1):227-40. DOI:10.1079/bjn19700023 |
- Kaufman S. A model of human phenylalanine metabolism in normal subjects and in phenylketonuric patients. Proc Natl Acad Sci U S A. 1999 Mar 16;96(6):3160-4. DOI:10.1073/pnas.96.6.3160 |
- Cortiella J, Marchini JS, Branch S, Chapman TE, and Young VR. Phenylalanine and tyrosine kinetics in relation to altered protein and phenylalanine and tyrosine intakes in healthy young men. Am J Clin Nutr. 1992 Sep;56(3):517-25. DOI:10.1093/ajcn/56.3.517 |