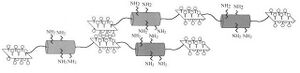
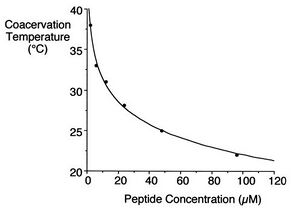
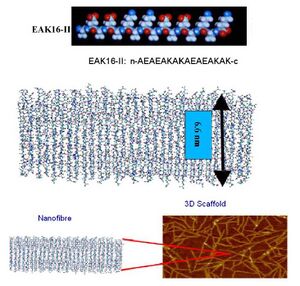
IGEM:IMPERIAL/2008/Prototype/Biomaterials
<html> <style type="text/css"> .firstHeading {display: none;} </style> </html> <html> <style type="text/css">
table.calendar { margin:0; padding:2px; }
table.calendar td { margin:0; padding:1px; vertical-align:top; } table.month .heading td { padding:1px; background-color:#FFFFFF; text-align:center; font-size:120%; font-weight:bold; } table.month .dow td { text-align:center; font-size:110%; } table.month td.today { background-color:#3366FF } table.month td {
border:2px; margin:0; padding:0pt 1.5pt; font-size:8pt; text-align:right; background-color:#FFFFFF; }
- bodyContent table.month a { background:none; padding:0 }
.day-active { font-weight:bold; } .day-empty { color:black; } </style> </html>
| <html><script language="JavaScript">
var timeout = 250; var closetimer = 0; var ddmenuitem = 0; // open hidden layer function mopen(id) { // cancel close timer mcancelclosetime(); // close old layer if(ddmenuitem) ddmenuitem.style.visibility = 'hidden'; // get new layer and show it ddmenuitem = document.getElementById(id); ddmenuitem.style.visibility = 'visible'; } // close showed layer function mclose() { if(ddmenuitem) ddmenuitem.style.visibility = 'hidden'; } // go close timer function mclosetime() { closetimer = window.setTimeout(mclose, timeout); } // cancel close timer function mcancelclosetime() { if(closetimer) { window.clearTimeout(closetimer); closetimer = null; } } // close layer when click-out //document.onclick = mclose; </script> | ||||||||||||||||
| <html><style type="text/css">
div.Section { font:11pt/16pt Calibri, Verdana, Arial, Geneva, sans-serif; background-image: url(http://openwetware.org/images/a/a0/Background.PNG); background-size: 100%; background-origin: content; } /* Text (paragraphs) */ div.Section p { font:11pt/16pt Calibri, Verdana, Arial, Geneva, sans-serif; text-align:justify; margin-top:0px; margin-left:30px; margin-right:30px; } /* Headings */ div.Section h1 { font:22pt Calibri, Verdana, Arial, Geneva, sans-serif; text-align:left; color:#3366FF; font-weight:bold; } /* Subheadings */ div.Section h2 { font:18pt Calibri, Verdana, Arial, Geneva, sans-serif; color:#3366FF; margin-left:5px; font-weight:bold; } /* Subsubheadings */ div.Section h3 { font:22pt Calibri, Verdana, Arial, sans-serif; color:#E5EBFF; margin-left:10px; font-weight:bold; } /* Subsubsubheadings */ div.Section h4 { font:22pt Calibri, Verdana, Arial, sans-serif; color:#2B48B3; margin-left:10px; font-weight:bold; } /* Subsubsubsubheadings */ div.Section h5 { font:12pt Calibri, Verdana, Arial, sans-serif; color:#3366FF; margin-left:20px; } /* References */ div.Section h6 { font:12pt Calibri, Verdana, Arial, sans-serif; font-weight:bold; font-style:italic; color:#3366FF; margin-left:25px; } /* Hyperlinks */ div.Section a { } div.Section a:hover { } /* Tables */ div.Section td { font:11pt/16pt Calibri, Verdana, Arial, Geneva, sans-serif; text-align:justify; vertical-align:top; padding:2px 4px 2px 4px; } /* Lists */ div.Section li { font:11pt/16pt Calibri, Verdana, Arial, Geneva, sans-serif; text-align:left; margin-top:0px; margin-left:30px; margin-right:0px; } /* TOC stuff */ table.toc { margin-left:10px; } table.toc li { font: 11pt/16pt Calibri, Verdana, Arial, Geneva, sans-serif; text-align: justify; margin-top: 0px; margin-left:2px; margin-right:2px; } /* [edit] links */ span.editsection { color:#BBBBBB; font-size:10pt; font-weight:normal; font-style:normal; vertical-align:bottom; } span.editsection a { color:#BBBBBB; font-size:10pt; font-weight:normal; font-style:normal; vertical-align:bottom; } span.editsection a:hover { color:#3366FF; font-size:10pt; font-weight:normal; font-style:normal; vertical-align:bottom; } /* Drop-down Menu */
margin: 0; padding: 0; z-index: 30 margin: 0; padding: 0; float: center; font: bold 12pt Calibri, Verdana, Arial, Geneva, sans-serif; border: 0px; list-style: none; }
display: block; margin: 0px 0px 0px 0px; padding: 0 0 12px 0; color: #FFFFFF; text-align: center; text-decoration: none; }
border: 0px }
position: absolute; visibility: hidden; margin: 0; padding: 0; background: #66aadd; border: 1px solid #66aadd } #sddm div a { position: relative; left: 0; display: block; margin: 0; padding: 5px 10px; width: auto; white-space: nowrap; text-align: left; text-decoration: none; background: #FFFFFF; color: #2875DE; font: 11pt Calibri, Verdana, Arial, Geneva, sans-serif } #sddm div a:hover { background: #66aadd; color: #FFFFFF } </style></html>
| ||||||||||||||||