IGEM:Harvard/2006/Cyanobacteria/Notebook/2006-10-9
PCR results from ligation
Not good... (no egels, reg. gel)
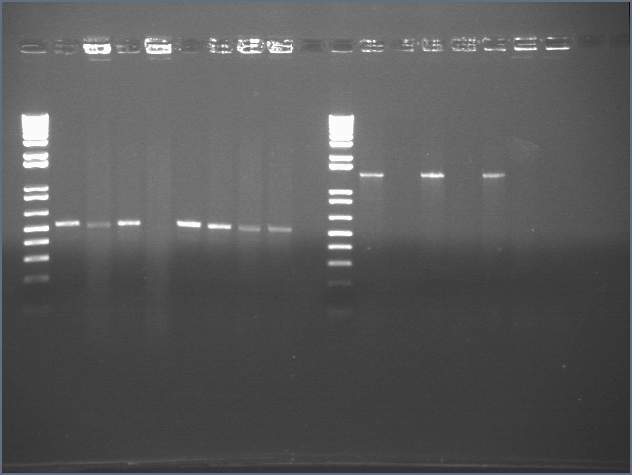
- Lane1: 1kb+
- Lane2-9: Samples 1-8, also known as KaiB+KaiC (J36011)
- Lane 11: 1kb+
- Lane 12-18: Samples A-G, also known as KaiA+KaiC(J36012)
Looks as if our theoretical J36011 did not ligate, and our theoretical J36012 self-ligated. These results mirror the failed ones on Nick's notebook: http://openwetware.org/wiki/IGEM:Harvard/2006/Nicholas_Stroustrup%27s_Notebook#Third_Round_of_ligations
Retry of ligation
- J36011 \SP + J36010 \XP
- J36012 \SP + J36010 \XP
For ligation, we use the Roche 5min quick ligase kit. Vials 2 and 1 are proprietary, but Vial 3 is generic T4 DNA ligase.
It is still unknown if a 1:3 volume ratio or a 1:3 molar ratio is ideal for ligations. I personally use the latter and would recommend that over the former. 1:1 may be good for small inserts, big vectors; we havent tested it though so we're not sure.
For J3011+J36010:
1.5 uL vector DNA @65.4ng/uL 3*(1600 /3750)* 98.1ng from above = 125.6 ng insert DNA @ 33.5 ng/uL = 3.75 uL 2uL 5X DNA dilution buffer 2.75uL dH20 10ul Ligation buffer 1ul ligase
For J3012+J36010:
1.5 uL vector DNA @60.6ng/uL 3*(1600 /3150)* 98.1ng from above = 149.5 ng insert DNA @ 33.5 ng/uL = 4.46 uL 2uL 5X DNA dilution buffer 2.04 uL dH20 10ul Ligation buffer 1ul ligase
- Make sure the reaction is 10uL!
- Also make sure the total amount of DNA does not exceed 200ng. Efficiency won't improve with that much DNA.
- USE the 5x DNA dilution buffer; do not skimp on it!
- Add 10uL tube 1, 1uL tube 3.
- Let sit at Room Temperature for 30min"
- USE only 2uL of ligation product
- Note: I used NEB T4 DNA ligase for the J36011 ligation as we ran out of Roche's, but theoretically it should be the same (I hope).
EDIT: Putting on hold, the pre-transformation post-ligated products a re in the freezer.
Genetic check of the KaiB and KaiA Nick made...
=(. Unless I am mistaken, the KaiA (J36012) is on the dot BUT the KaiB (J36011) is an empty plasmid. Meaning either we picked the wrong one or the numbering system was incorrect.