User:Perry/Spring 2006 pCMV-Tag2A / hDlg work
Notes from fall 2005 have been archived into "Perry, fall 2005"
Table 1: Composition of the hDlg constructs
| Construct | Parts |
|---|---|
| LS25G | P1 + P2 |
| LS35G | P1 + P3 |
| LS325G | P1 + P4 + P5 |
| LS254G | P1 + P6 + P7 + P8 |
| LS354G | P1 + P4 + P9 + P8 |
Table 2: Composition and construction of parts
| Parts | Domains | Template | Primers |
|---|---|---|---|
| P1 | L27 | F11 | L27S + L27A |
| P2 | SH3-I2-I5-GK | SG25 | SH3S + GKA |
| P3 | SH3-I3-I5-GK | SG35 | SH3S + GKA |
| P4 | SH3-I3 | SG35 | SH3S + I3A |
| P5 | I2-I5-GK | SG25 | I2S + GKA |
| P6 | SH3 | F11 | SH3S + SH3A |
| P7 | I2-I5-I4 | I2-I5-I4/Topo | I2S + I4A |
| P8 | GK | F11 | GKS + GKA |
| P9 | I5-I4 | I2-I5-I4/Topo | I5S + I4A |
3/3/06
Transformed DH5alpha competent cells (50 ul aliquots) with pcMV-Tag2A vectors. The stock received was 1 ug/ul, used 1 ul for transformation (did two identical ones), 2.5 ul pUC19 for (+) and nothing for (-). On ice for 30 minutes. Heat shock 42 degrees Celsius for 25 seconds. On ice for 2 minutes. Added 950 ul prewarmed SOC, and shook at 225 rpm, 37 degrees, for 1 hour. (followed protocol from DH5alpha product information, except for heat shock step)
Transformed TOP10 competent cells with hDlg-cloned Topo vectors SG25, SG35, and F11. Diluted stock vector 1:10, used 5 ul for transformation, 2.5 ul pUC19 for (+), nothing for (-). On ice for 30 minutes. Heat shock 42 degrees Celsius for 30 seconds, on ice for 2 minutes. Added 250 ul room-temp SOC, and shook at 225 rpm, 37 degrees for 1 hour. (followed previous MCB100 protocol)
Plated bacteria with controls and Topo vectors on carb plates, bacteria with pCMV vectors on kan plates, with glass beads under burner. Placed in 37 degree incubator at 6 pm.
3/4/06
Transformations didn't go great, so Yi-an reperformed them.
3/5/06
hDlg transformations from yesterday didn't show identifiable colonies, more a smear of nothing. pCMV transformations from yesterday produced a few colonies. hDlg transf from Friday were overgrown in SG35 and F11; must have used too much vector to transform.
Prepared three 200 ml LB liquid cultures from single colonies of bacteria transformed with hDlg F11, SG25, and SG35, with 200 ul ampicillin (50 mg/ml); and one 200 ml, pCMV, with 200 ul kanamycin (50 mg/ml). put in shakers around 9:30 pm, set at 300 rpm and 37 degrees Celsius. also streaked onto new plates.
3/10/06
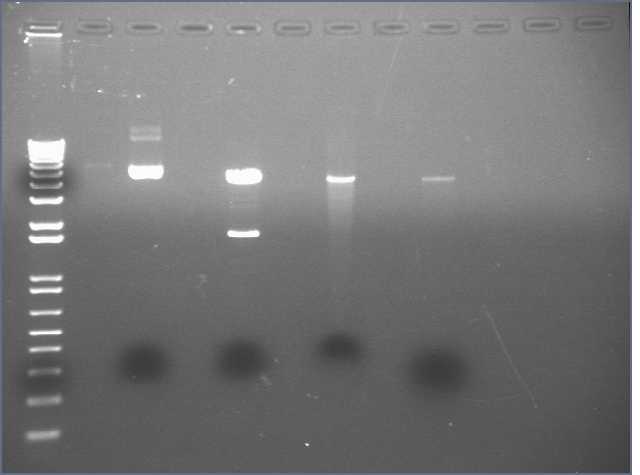
Yi-an already performed the midipreps and PCRs. Today I PCR purified P5-9. Before purification, I took out 10 ul fromeach and added 10 ul water for 1.2% E-gel. I measured the volume left and added 5 volumes Buffer PB to each PCR product. P5 had 20 ul, and I added 100 ul PB; P6, 35, 175; P7, 35, 175; P8, 30, 150; P9, 35, 175. I proceeded with PCR purification, eluting with 30 ul EB. I took out 10 ul from each purified product and added 10 ul water for E-gel. I also took out 10 ul of 1 kb ladder and of 2 log ladder, and added 10 ul water for E-gel.
Lane 1: 1 kb ladder
Lane 2: P5 before
Lane 3: P5 after
Lanes 4-11: P6-9 before and after
Lane 12: 2 log ladder
P5 and P6 have the right lengths.
Setup PCRs for P7-9 again, but this time with undiluted I2-I5-I4/Topo for 7 and 9. Repeated E-gel with 10 ul before and after PCR purification. The volume left for PCR purification was 37 ul in each, and I added 185 ul PB to each.
Lane 1: 1 kb ladder
Lane 2: P7 before
Lane 3: P7 after
Lanes 4-7: P8 and P9 before and after
Lane 8: 2 log ladder
Performed Topo vector ligation using P5, P6, and P8, with 4 ul PCR product, 1 ul Salt solution, 1 ul Topo vector. Incubated room temp 5 min, transferred into tube of chemically competent Top10 cells. Split one 60 ul tube into two 30 ul tubes, one for untreated (-) and one for pUC19-transformed positive (+). On ice for 20 minutes, heat shock at 42 degrees Celsius for 30 seconds, one ice for 2 minutes, added 250 ul SOC media to each tube, shook at 37 degrees Celsius for 1 hour, plated w/ carb.
3/13/06
Transformed one tube of Top10 with 2 ul of original SG35-cloned vector, left one tube untreated for negative. Used 350 ul SOC, and plated a 50, 100, and 200 ul plate from each tube.
Prepared 2 identical double digests of pCMV midiprep: 5 ul pCMV (~213 ng/ml), 2 ul BamHI buffer (10X), 0.2 ul BSA (100X), 0.5 ul BamHI, 0.5 ul XhoI, 11.8 water. Left in PTC-100 at 37 degrees for 6 hours, 80 degrees for 20 minutes, 4 degrees indefinitely.
3/14/06
Ran digest on gel.
Lane 1: 1 kb ladder
Lane 3: pCMV digest #1
Lane 5: pCMV digest #2
The upper band is the correct size for linear vector, and the lower band is probably supercoiled undigested vector.
Performed 2 parallel QIAEX II gel extractions, for DNA >4kb. Preweighed centrifuge tubes at 1.016 and 1.030 g, then weighed with gel slices at 1.108 and 1.161 g. Used 300 and 390 ul QX1, 30 ul QIAEX II each b/c of strong bands, but I forgot to add 2 volumes of water to each. After 50 degree incubation, I added 10 ul 3M sodium acetate to each because of a pinkish color which then turned yellow. Air dried over 30 minutes until about half of each pellet turned from clearish gray to white. Eluted each twice with 20 ul water. Ended up with 2 tubes of ~35 ul elution. Froze one tube, used other tube for a redigestion.
Prepared 3 digests.
1. New digest of pCMV, same as yesterday except with 3 ul DNA and 13.8 ul water.
2. A negative control digest with 3 ul DNA, no enzyme, and 14.8 ul water.
3. A redigest of the DNA that was gel extracted today, using 35 ul gel extraction, 5 ul BamHI buffer, 0.5 ul BSA, 1.25 ul BamHI, 1.25 ul XhoI, and 7 ul water, for a 50 ul digestion.
Ran digest at 37 degrees for 12 hours, 80 degrees for 20 minutes, and 4 degrees indefinitely.
Had to pause the digestion at 3 hours and use PTC machine for annealing BB linkers. 5 ul BBCS1 (for 50 pmol from 10 uM), 5 ul BBCS2, 5 ul T4 ligase buffer (10X), 0.5 ul T4 kinase, 34.5 ul water, at 37 degrees for 30 minutes, 65 degrees for 20 minutes, 94 degrees for 1 minute, let cool to room temperature.
Left digests out at room temp. Returned to PTC after linkers were done and completed 9 more hours of 37 degree incubation.
Helped Yi-an with gel extraction of PCR products. Repeated steps for QUIAEX II protocol, only used 3 volumes of QX1, 10 ul of QIAEX II, added 10 ul sodium acetate two times until yellow. Eluted twice with 20 ul water.
Performed Topo cloning with 1 ul vector, 1 ul salt solution, 4 ul gel-purified PCR product. Transformed Top10 cells and plated onto new amp plates which Alain poured.
3/15/06
Yi-an ran gel of digests for me.
Lane 1: 1 kb
Lane 3: Undigested vector
Lane 5: New digest with 3 ul midiprep
Lane 7: Redigest of gel extracted DNA
Lane 9: Gel extracted DNA.
The 2nd band appeared again, and did not match up with supercoiled undigested plasmid. So we suspect that this is an actual insert from the plasmid. pCMV-Tag2 control plasmid (for transfection control) includes an Luciferase insert. Cut out bands from Lanes 5, 7, and 9, and froze.
Yi-an performed two transformations using DNA from tubes labelled pCMV-Tag2A and pCMV-Tag2control.
Topo cloning/transformation with the gel-purified PCR products produced colonies. Yi-an prepared inoculations and made new streaks from four different colonies on each transformation plate. (P1-, P2-, P3-, P4-, P5-, P6-, P8-1 through 4)
3/16/06
Alain spun down pellets from inoculations. I performed minipreps. I spilled the Buffer P1 resuspension of the Part 2-2 pellet on the bench, pipet it off the bench and back into the tube, and proceeded normally with the miniprep. I prepared EcoRI digests from all the minipreps: 10 ul DNA, 2.5 ul EcoRI buffer, 0.25 ul BSA, 0.625 ul EcoRI, 11.625 water, for a 25 ul digest. Left in PTC at 37 degrees for 12 hours, 80 degrees for 20 minutes, 4 degrees indefinitely.
Ran gel of digests.
Lane 1: 1 kb ladder
Lanes 2-5: P1-1 through 4
Lanes 6-9: P2-2 through 4
Lanes 10-13: P3-3 through 4
Lanes 14-15: P4-1 and 2
Lane 1: 1 kb ladder
Lanes 2-3: P4-3 and 4
Lanes 4-7: P5-1 through 4
Lanes 8-11: P6-1 through 4
Lanes 12-15: P8-1 through 4
The bands that appeared for P1, 4, 5 are the correct size. No bands appeared for P2 and 3. The bands for P6 are the incorrect size.
3/19/06
Repeated pCMV transformation using Tag2A and Tag2, with DH5alpha competent cells. Followed the issued protocol. Plated 200 ul from the 1ml SOC mixtures and refrigerated the rest.
3/20/06
Inoculated Tag2 and Tag2A from colonies (ran toothpick across several colonies from each plate) with 3ml LB, 2ul kan. Ran Yi-an's digests on gel. They were EcoRI digests of DNA miniprepped from inoculations of four more colonies from the latest plates of transformations with Topo vectors cloned with PCR products.
Lane 1: 1 kb plus ladder
Lanes 2-5: P2-5 through 8
Lanes 6-9 P3-5 through 8
Lanes 10-13: P5-5 through 8
Lanes 14-17: P6-5 through 8
Lane 18: 1 kb plus ladder
3/24/06
Gel extracted from slices that Alain excised yesterday of digested pCMV Tag2A. One slice came from two lines, and the other slice came from one lane. Performed two parallel extractions, eluted each twice with 20ul water, and collected elutions into one tube.
Performed three ligation/transformations using digested pCMV Tag2A vector which I had just gel-extracted, and BB linker insert created 3/14/06: (1) 5ul insert, 5ul vector, (2) 2.5ul insert, 7.5ul vector, (3) 7.5ul insert, 2.5ul vector. But I absentmindedly used Top10 cells. Alain realized my mistake right after addition of SOC. Great. Repeated the three ligation/transformations using DH5alpha cells.
Purified product from Alain's PCR reactions #1-6 from yesterday. Eluted with 30ul EB, except accidentally used 50ul for #1, and lost a drop from the PB/PCR mixture from #6.
Alain first PCRed out I2I5I4 from a cDNA library using primers C and BL16. Then he PCRed the product with our primers. #1 and 2 are same PCR reaction with primers I2S and I4A. #3 and 4 are I5S and I4A. #5 and 6 are I3S and I4A.
TOPO cloning: 4ul purified PCR product, 1ul salt solution, 1ul Topo vector. Used PCR purified products #2, 3, 5. Transformation with Top10 cells, 250ul SOC, on carb plates.
3/25/06
Transformation plates of Topo clonings grew well, almost too well. An e-gel Alain ran of the PCR purifications of #2, 3, 5 showed no bands. We suspect that the cDNA plasmids somehow got through the PCR purifications. We plan to do only gel isolations from now on.
Transformation plates of pCMVTag2A-BB linker did not grow. The DH5alpha protocol calls for 1mL initial growth mixture, plating 200ul, and refrigerating the rest. I plated the remaining ~800ul onto new plates and left in incubator. Alain will take them out on Monday.
4/4/06
Prepared PCR reaction with 44ul Platinum PCR Supermix, 2ul P1TagR primer (10uM), 2ul L27TagS primer (10uM), and 2ul P1-1a miniprep (1:100). Used BB4 program in PCR machine #6.
Performed midiprep of pCMVTag2A. Eluted with 500ul water, yielded 424 ng/ul. Used 2ul in a digest: 14.8ul water, 2ul DNA, 0.2 BSA (100X), 2ul BamHI buffer, 0.5ul BamHI, 0.5ul XhoI. Ran digest at 37 degrees for 12 hours, 80 degrees for 20 minutes, 4 degrees indefinitely.
4/5/06
Ran gel.
Lane 1: 1 kb plus (10ul)
Lane 3: P1-1a 1:100 (10ul+3ul dye)
Lanes 5-6: P1-1a PCR (50ul+3ul dye, split into two wells)
Lane 8: pCMV midiprep (1ul+3ul dye)
Lane 9: pCMV digest (20ul+3ul dye)
Cut out bands from lanes 5-6 and 9, and gel extracted.
Ligated digested pCMV (3ul) with BB linker (7 ul). Transformed with XL1 Blue.
Topocloned P1-1a PCR product. Transformed with OneShot.
Prepared 200ml cultures of P1-1a and P2-3a for midiprep.
4/6/06
Performed midiprep of P1-1a (~350ng/ul) and P2-3a (~150ng/ul).
Inoculated and streaked from four colonies of Topocloned/transformed P1-1a* PCR product (using P1TagR and L27TagS primers, missing upstream BioBricks site).
pCMV-BBlinker ligation/transformation yielded a few very small colonies. Inoculated and streaked from four colonies.
Inoculated and streaked four more colonies (9a-12a) from Alain's last P3 Topocloning/transformation. 1a-4a yielded bands of incorrect size, and 5a-8a showed no insert.
Sent out sequencing reactions for P5-1a through 4a, P7-2a, P8-1a through 3a, and P9-1a, 3a, 4a.
4/10/06
Ran e-gel of digests from Friday.
Lane 1: 1 kb plus
Lane 2: BB1 undigested
Lanes 3-10: BB1a, 1b, 2a, 2b, 3a, 3b, 4a, 4b
Lane 11: 1 kb plus
Lane 12: Water
Lane 1: 1 kb plus
Lanes 2-5: P1-1a* (1)-(4)
Lanes 6-9: P3-9a, 10a, 11a, 12a
Lane 10: 1 kb plus
Lanes 11-12: Water
Treated pCMV digest with calf intestinal phosphatase (measured 35ul digest, added 5ul 10x buffer, 1ul CIP, 9ul water). Incubated 37dC for 1 hour, added 2ul 0.5M EDTA (brochure suggests 1/10 volume 200mM EGTA), then incubated 65dC for 10 min. Performed PCR purification.
Alain sent in BB1-4 and P3-9a, 11a, 12a in for sequencing.
Repeated ligation with 5ul CIP-treated pCMV digest and 5ul BB linker, then transformation with XL1 cells.
Repeated P1-1a* Topo cloning and transformation.
4/11/06
Performed midiprep of P8-2a and P5-4a which Alain has sequenced already.
pCMV-BB transformation did not yield any colonies. Left plate in incubation. Plated the refrigerated leftovers and left in incubation.
P1-1a* yielded many colonies. Streaked and prepared inoculations from 8 colonies.
4/12/06
Performed minipreps of inoculations. Prepared 25 ul EcoRI digests of each: 10 ul DNA, 2.5 ul EcoRI buffer, 0.25 ul BSA, 0.625 ul EcoRI, 11.625 water. (first made a master mix enough for 10).
Also prepared XbaI/PstI digests of midipreps of parts that have been sequenced. (P1-1a, P2-3a, P4-4, P5-4a, P6-4, P8-2a): 10ul DNA, 5ul buffer3, 0.5ul BSA, 1.25ul XbaI, 1.25ul PstI, 32ul water.
4/13/06
Ran gel of digests.
Lanes 1, 10: 1 kb plus
Lanes 2-9: P1-1a* #1-8 (4/12 miniprep)
Still no inserts. Have abandoned the P1-1a* effort b/c it seems we'll have the other parts ready.
Lanes 1, 15: 1 kb plus
Lane 3: P1-1a XbaI/PstI digest
Lane 5: P2-3a
Lane 7: P4-4
Lane 9: P5-4a
Lane 11: P6-4
Lane 13: P8-2a
The bottom band is the insert of interest in each case. They are all the expected sizes, but the band intensities/sizes are disappointing since we used 10ul of a midiprep. Nevertheless, bands were still excised and extracted.
4/17/06
Yi-an performed midipreps and digests over the weekend for BB3, P3, P7, P9. I treated the BB3 digest with CIP (measured 52ul, added 6ul buffer, 1ul CIP, 1ul water) for 1 hour at 37dC.
Lanes 1,11: 1 kb plus
Lane 3: P3 XbaI/Pst digest
Lane 5: P9
Lane 7: P7
Lane 9: BB3 SpeI/PstI digest
P9 and P7 yielded very faint bands, which I excised and froze.
It is difficult to separate the insert band from the P3 digest b/c the TopoII vector has an XbaI site of its own. The distance from the BioBricks XbaI site and TopoII's XbaI is about 1 kb, which happens the be the size of the P3 insert. The BB3 linearized vector should be around 4kb, which is hard to discern in the smear. There may have been too much DNA used in the digest. Yi-an used original midiprep.
Prepared new XbaI/PstI digests of P1-P9 and SpeI/PstI digest of BB, using 1:10 dilutions of the midipreps. Also prepared an XbaI single digest of P3 using 1:10 diluted midiprep, and a SpeI single digest of BB using 1:10 diluted midiprep.
The single digests need to be gel isolated and extracted, and then redigested using PstI (Buffer3). The double digests can be excised and frozen; they don't need to be extracted immediately since these are just for backup.
5/2/06
Wow, back in the lab.
Ran gel of Yi-an's digests from earlier today.
Lanes 1,12: 1 kb plus
Lanes 3-4: P1-P4-P5 XbaI/PstI
Lanes 6-7: P1-P4-P9 EcoRI/SpeI
Lanes 9-10: P1-P6-P7 EcoRI/SpeI
Ran e-gel of Yi-an's P1-1a digests from earlier today.
Lane 1,6: 1 kb plus
Lane 2: P1-1a EcoRI
Lane 3: P1-1a SpeI
Lane 4: P1-1a EcoRI/SpeI
Lane 5: P1-1a untreated
Cut out bands for P1-P4-P5, P1-P4-P9, and P1-P6-P7. Performed QiaexII gel extraction. Performed ligations: P1-P4-P5 (X/P) with pCMV BB (S/P), P1-P4-P9 (E/S) with P8 (X/P), P1-P6-P7 (E/S) with P8 (X/P). Froze P1-P4-P5-pCMVBB ligation to save for transformation tomorrow. Prepared PCR reactions of P1-P4-P9-P8 and P1-P6-P7-P8 ligations using primers L27S and GKA; incubated using program A in PCR machine 6. Performed PCR purification. Used 10ul of purified PCR in 50ul XbaI/PstI digests. Incubated for 6 hours. (Accidentally aliquotted 10ul PCR reaction into digest mixtures before purification.)
5/3/06
Ran gel.
Lanes 1, 9: 1 kb plus
Lanes 3-4: P1-P4-P9-P8 XbaI/PstI
Lanes 6-7: P1-P6-P7-P8 XbaI/PstI
Excised bands, gel extracted. Ligated 5ul of each extraction with 5ul pCMV BB (S/P).
Transformations into XL1 competent cells.
1. P1-P2-pCMVBB (5/1 ligation)
2. P1-P2-pCMVBB (5/2 ligation)
3. P1-P3-pCMVBB (5/2 ligation)
4. P1-P4-P5-pCMVBB (5/2 ligation)
5. P1-P4-P9-P8-pCMVBB (5/2 ligation)
6. P1-P6-P7-P8-pCMVBB (5/2 ligation)
5/4/06
I got no colonies except for a bunch in (+) and one colony in P1-P6-P7-P8. I prepared a streak and a liquid culture from this colony.