|
Objective
- Measure pH of Dye solutions from 2/22 (first dye reaction), and compare with pH of dyed solutions from 3/20 (second dye reaction) to see if pH can account for the inconsistencies in emission data.
- Take UV-Vis and Fluorescence spectra of four control solutions:
1. 70 Au/BSA ratio solution made on 2/28
2. 166 Au/BSA ratio solution made on 2/28
3. BSA/HCl solution made on 3/20
4. Dye in Water
Description
- First Dye Reaction Solutions (2/22)
--Measure initial pH:
1. 70 Au/BSA ratio solution with dye: initial pH = 8.12
2. 166 Au/BSA ratio solution with dye: initial pH = 8.43
3. BSA/HCl solution with dye: initial pH = 8.3
--Increase the pH of 70 Au/BSA ratio dye solution by adding Tris buffer:
70 Au/BSA ratio solution with dye: add 10 drops, final pH = 8.30
- Second Dye Reaction Solutions (3/20)
--Measure the initial pH:
1. 70 Au/BSA ratio solution with dye: initial pH = 8.00 (last week pH = 8.07)
2. 166 Au/BSA ratio solution with dye: initial pH = 8.35 (last week pH = 8.13)
3. BSA/HCl solution with dye: initial pH = 7.98 (last week pH = 8.09)
--Increase the pH of dye solutions by adding Tris buffer:
1. 70 Au/BSA ratio solution with dye: add 6 drops, final pH = 8.29
2. 166 Au/BSA ratio solution with dye: add 3 drops, final pH = 8.41
3. BSA/HCl solution with dye: add 7 drops, final pH = 8.28
- Fluorescence and UV-Vis Studies
--Centrifuge all six dye solutions (5 minutes, 13,200 rpm)
--Take Fluorescence spectrum of six dye solutions: Excitation = 600nm; emission = 620 to 800nm
--Take Fluorescence spectrum of the four control solutions.
1. 166 Au/BSA ratio solution
2. 70 Au/BSA ratio solution
3. BSA/HCl solution
4. Dye in solution
--Take UV-Vis spectrum of all ten solutions (200 - 800 nm)
Data
- Fluorescence Spectra : Second Dye Reaction (3/20) Solutions

- Fluorescence Spectra: First Dye Reaction (2/22) Solutions

- Fluorescence Spectra: Control Solutions (No Dye)

- UV-Vis Spectra: Second Dye Reaction (3/20) Solutions


- UV-Vis Spectra: First Dye Reaction (2/22) Solutions


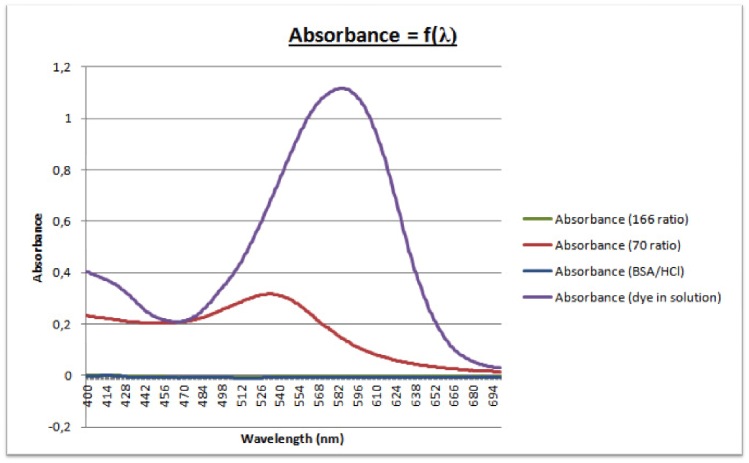
- UV-Vis Spectra: Control Solutions (No Dye)

Notes
- Tris buffer was added to the 70 Au/BSA ratio dye solution in the first dye reaction and all three dye solutions from the second dye reaction. Because the reactions are pH sensitive, making the pH's of the solutions equal could provide more consistent Fluorescence spectra between the solutions in the two dye reactions.
- After making the pH's of the solutions similar between the two dye reactions, the fluorescence data was more consistent with the 70 Au/BSA ratio solution having the highest emission peak in both spectra. The UV-Vis data remained consistent between the two dye reactions.
- The pH of the second dye reaction solutions changed since the previous week. This can be attributed to contaminants (ex. water used to clean tubes, pH meter), or to a reaction that is occurring in the dye solutions and affecting the pH. The pH meter was calibrated before each set of measurements, therefore instrumental error should be minimal.
|  Chem-571
Chem-571








