Objective
- Measure protease Trypsin's kinetics with the fluorescence assay using 1nM of trypsin at different heating intervals.
- Create a calibration curve using fluorescence for trypsin.
Procedure
The general protocol detailed in Dr. Hartings' lab notebook was used. The following specific steps were performed:
- Used eppendorf tube no. 6 that weighed (1.01922 )g, and contained (0.00128)g of trypsin.
- Added 1mL of phosphate buffer.
- Final concentration: (54.93 µM
- We diluted the 54.93 uM )Trypsin sample by pipetted 0.0182 mL to 0.982 ml of phosphate buffer to make 1 uM solution of Trypsin.
- We pipetted 0.01 mL to make 0.01 uM solution of Trypsin.
- Used 7 eppendorf tubes, each containing gold fibers.
- To each tube add:
- 0.9981mL of buffer
- 0.0019mL of trypsin (add this at the time of putting the tubes in the 37˚C hot water bath).
- In on eppendorf tube add:
- 0.9981mL of phosphate buffer
- 0.0019mL of trypsin solution
- Additional specifications:
- Prior to prepping the samples, the eppendorf tubes containing the fibers were centrifuged for 10 mins at 300rpm.
- After heating the samples, they were all centrifuged for 1 min. at 13'200rpm. This was done only for the samples, not the blanks.
Calculations:
V1 = [(0.1µM)(1mL)]/52.36µM = 0.0019mL, amount of trypsin solution needed
Volume of buffer: 1mL - 0.0019mL = 0.9981mL
Data
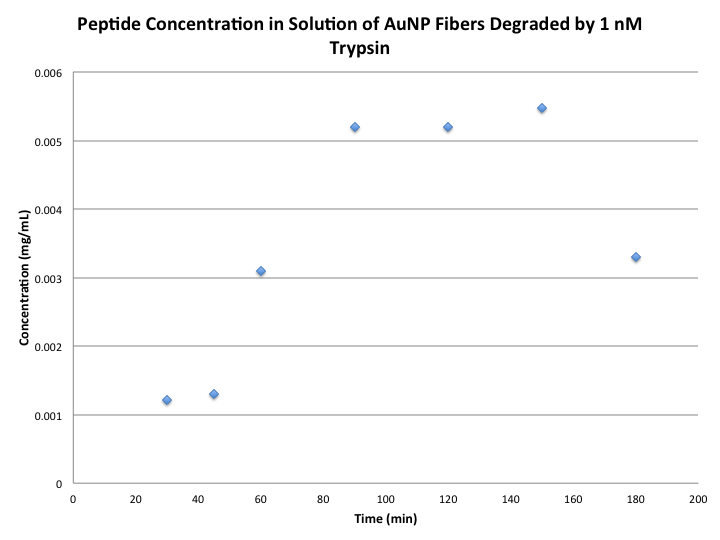
|
