Nuclear Gene Adenita Culture 454 Amplification
Re-Amp Instagene Culture 57, A, B, C, D, E, F, G, H, I
| EX Takara RXN Mixture
|
conc
|
1X
|
5
|
'
|
'
|
| Milli Q water |
|
4.15 |
20.75 |
|
|
| Takara EX Buffer |
10X |
1 |
5 |
|
|
| dNTP |
2.5uM |
0.8 |
4 |
|
|
| Primer Mix |
20uM |
1 |
|
|
|
| EX Taq |
5U |
0.05 |
0.25 |
|
|
| Template |
Instagene |
3 |
15 |
|
|
|
|
|
45 |
|
|
|
|
|
9.0ul / RXN add 1.0ul Primer |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| PCR cycle |
|
|
|
|
|
| 98C |
10 sec |
|
|
|
|
| 60C |
1 min |
|
|
|
|
| x35 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| AMP Lane |
Template |
Primer |
AMP Lane |
Template |
Primer
|
| 1 |
57 Instagene |
B9 |
31 |
F Instagene |
B10
|
| 2 |
57 Instagene |
C9 |
32 |
F Instagene |
C10
|
| 3 |
57 Instagene |
D9 |
33 |
F Instagene |
D10
|
| 4 |
57 Instagene |
E9 |
34 |
F Instagene |
E10
|
| 5 |
57 Instagene |
F9 |
35 |
F Instagene |
F10
|
| 6 |
A Instagene |
B5 |
36 |
G Instagene |
B11
|
| 7 |
A Instagene |
C5 |
37 |
G Instagene |
C11
|
| 8 |
A Instagene |
D5 |
38 |
G Instagene |
D11
|
| 9 |
A Instagene |
E5 |
39 |
G Instagene |
E11
|
| 10 |
A Instagene |
F5 |
40 |
G Instagene |
F11
|
| 11 |
B Instagene |
B6 |
41 |
H Instagene |
B12
|
| 12 |
B Instagene |
C6 |
42 |
H Instagene |
C12
|
| 13 |
B Instagene |
D6 |
43 |
H Instagene |
D12
|
| 14 |
B Instagene |
E6 |
44 |
H Instagene |
E12
|
| 15 |
B Instagene |
F6 |
45 |
H Instagene |
F12
|
| 16 |
C Instagene |
B7 |
46 |
I Instagene |
B1
|
| 17 |
C Instagene |
C7 |
47 |
I Instagene |
C1
|
| 18 |
C Instagene |
D7 |
48 |
I Instagene |
D1
|
| 19 |
C Instagene |
E7 |
49 |
I Instagene |
E1
|
| 20 |
C Instagene |
F7 |
50 |
I Instagene |
F1
|
| 21 |
D Instagene |
B8 |
51 |
Neg Control |
B9
|
| 22 |
D Instagene |
C8 |
52 |
Neg Control |
C9
|
| 23 |
D Instagene |
D8 |
53 |
Neg Control |
D9
|
| 24 |
D Instagene |
E8 |
54 |
Neg Control |
E9
|
| 25 |
D Instagene |
F8 |
55 |
Neg Control |
F9
|
| 26 |
E Instagene |
B9 |
56 |
Pos Control |
B9
|
| 27 |
E Instagene |
C9 |
57 |
Pos Control |
C9
|
| 28 |
E Instagene |
D9 |
58 |
Pos Control |
D9
|
| 29 |
E Instagene |
E9 |
59 |
Pos Control |
E9
|
| 30 |
E Instagene |
F9 |
60 |
Pos Control |
F9
|
|
|
| *Positive Control = AV 20ng
|
| ** Extraction Control = AV Chelex
|
Load 2.0ul

Nuclear Gene Adenita Culture 454 Amplification
Culture 29, 31-43, 50
1. Adenita cultures 5-15 individuals.
2. Spin for 1 min at 12-14,000 rpm. Remove supernatant leaving 20-30ul, careful not to disturb pellet.
3. Add 100ul 20% chelex (Or add chelex until ~1/2 tube full of beads). Vortex, then quick spin.
4. Boil for 10 minutes.
5. Before use, vortex, then spin at 13000 rpm for 3 minutes. Use supernatant right above beads.
| EX Takara RXN Mixture
|
conc
|
1X
|
5
|
'
|
'
|
| Milli Q water |
|
4.15 |
20.75 |
|
|
| Takara EX Buffer |
10X |
1 |
5 |
|
|
| dNTP |
2.5uM |
0.8 |
4 |
|
|
| Primer Mix |
20uM |
1 |
|
|
|
| EX Taq |
5U |
0.05 |
0.25 |
|
|
| Template |
Chelex |
3 |
15 |
|
|
|
|
|
45 |
|
|
|
|
|
9.0ul / RXN add 1.0ul Primer |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| PCR cycle |
|
|
|
|
|
| 98C |
10 sec |
|
|
|
|
| 60C |
1 min |
|
|
|
|
| x35 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| AMP Lane |
Template |
Primer |
AMP Lane |
Template |
Primer
|
| 1 |
29 Chelex |
B5 |
46 |
39 Chelex |
B3
|
| 2 |
29 Chelex |
C5 |
47 |
39 Chelex |
C3
|
| 3 |
29 Chelex |
D5 |
48 |
39 Chelex |
D3
|
| 4 |
29 Chelex |
E5 |
49 |
39 Chelex |
E3
|
| 5 |
29 Chelex |
F5 |
50 |
39 Chelex |
F3
|
| 6 |
31 Chelex |
B7 |
51 |
40 Chelex |
B4
|
| 7 |
31 Chelex |
C7 |
52 |
40 Chelex |
C4
|
| 8 |
31 Chelex |
D7 |
53 |
40 Chelex |
D4
|
| 9 |
31 Chelex |
E7 |
54 |
40 Chelex |
E4
|
| 10 |
31 Chelex |
F7 |
55 |
40 Chelex |
F4
|
| 11 |
32 Chelex |
B8 |
56 |
41 Chelex |
B5
|
| 12 |
32 Chelex |
C8 |
57 |
41 Chelex |
C5
|
| 13 |
32 Chelex |
D8 |
58 |
41 Chelex |
D5
|
| 14 |
32 Chelex |
E8 |
59 |
41 Chelex |
E5
|
| 15 |
32 Chelex |
F8 |
60 |
41 Chelex |
F5
|
| 16 |
33 Chelex |
B9 |
61 |
42 Chelex |
B6
|
| 17 |
33 Chelex |
C9 |
62 |
42 Chelex |
C6
|
| 18 |
33 Chelex |
D9 |
63 |
42 Chelex |
D6
|
| 19 |
33 Chelex |
E9 |
64 |
42 Chelex |
E6
|
| 20 |
33 Chelex |
F9 |
65 |
42 Chelex |
F6
|
| 21 |
34 Chelex |
B10 |
66 |
43 Chelex |
B7
|
| 22 |
34 Chelex |
C10 |
67 |
43 Chelex |
C7
|
| 23 |
34 Chelex |
D10 |
68 |
43 Chelex |
D7
|
| 24 |
34 Chelex |
E10 |
69 |
43 Chelex |
E7
|
| 25 |
34 Chelex |
F10 |
70 |
43 Chelex |
F7
|
| 26 |
35 Chelex |
B11 |
71 |
50 Chelex |
B2
|
| 27 |
35 Chelex |
C11 |
72 |
50 Chelex |
C2
|
| 28 |
35 Chelex |
D11 |
73 |
50 Chelex |
D2
|
| 29 |
35 Chelex |
E11 |
74 |
50 Chelex |
E2
|
| 30 |
35 Chelex |
F11 |
75 |
50 Chelex |
F2
|
| 31 |
36 Chelex |
B12 |
76 |
Extraction Control |
B5
|
| 32 |
36 Chelex |
C12 |
77 |
Extraction Control |
C5
|
| 33 |
36 Chelex |
D12 |
78 |
Extraction Control |
D5
|
| 34 |
36 Chelex |
E12 |
79 |
Extraction Control |
E5
|
| 35 |
36 Chelex |
F12 |
80 |
Extraction Control |
F5
|
| 36 |
37 Chelex |
B1 |
81 |
Neg Control |
B5
|
| 37 |
37 Chelex |
C1 |
82 |
Neg Control |
C5
|
| 38 |
37 Chelex |
D1 |
83 |
Neg Control |
D5
|
| 39 |
37 Chelex |
E1 |
84 |
Neg Control |
E5
|
| 40 |
37 Chelex |
F1 |
85 |
Neg Control |
F5
|
| 41 |
38 Chelex |
B2 |
86 |
Pos Control |
B5
|
| 42 |
38 Chelex |
C2 |
87 |
Pos Control |
C5
|
| 43 |
38 Chelex |
D2 |
88 |
Pos Control |
D5
|
| 44 |
38 Chelex |
E2 |
89 |
Pos Control |
E5
|
| 45 |
38 Chelex |
F2 |
90 |
Pos Control |
F5
|
|
|
| *Positive Control = AV 20ng
|
| ** Extraction Control = AV Chelex
|
Load 2.0ul

LCO/HCO Amplification
| EX Takara RXN Mixture
|
conc
|
1X
|
6
|
'
|
| Milli Q water |
|
10.3 |
61.8 |
|
| Takara EX Buffer |
10X |
2 |
12 |
|
| dNTP |
2.5uM |
1.6 |
9.6 |
|
| Primer Mix |
20uM |
2 |
12 |
|
| EX Taq |
5U |
0.1 |
0.6 |
|
| Template |
Instagene |
4 |
|
|
|
|
|
96 |
|
|
|
|
16.0ul / RXN add 4.0ul Template |
|
|
|
|
|
|
| PCR cycle |
|
|
|
|
| 94C |
3 min |
|
|
|
| 94C |
10 sec |
|
|
|
| 45-68C |
3 min |
|
|
|
| RAMP 0.5C/sec |
|
|
|
|
| x35 |
|
|
|
|
| 68C |
5 min
|
| AMP Lane
|
Template
|
'
|
Sample Number
|
| 1 |
E. Windsor |
B2 |
8
|
| 2 |
Ithaca 1 |
A1 |
12
|
| 3 |
Woods Hole 1 |
C4 |
G
|
| 4 |
Neg Control |
|
|
| 5 |
AV Positive Control |
|
|
Load 2.0ul

454 library preparation (Alien Genes Mix)
Purification of adapter ligated sample by AMPure beads (modified protocol by Lennon et al. 2010)
1. Add 25ul of AMPure beads (x0.5) to 50ul of sample.
2. Pipette 10 times.
3. Incubate for 5 min at r.t.
4. Incubate the mixture on the magnet plate for 5 min at r.t.
5. Transfer the supernatant to a new 0.2ml tube.
6. Add 200 of 70% EtOH to the beads (for <300bp).
7. Add 53.2ul of AMPure (x0.7) beads to the supernatant.
8. Repeat steps 2 to 6 (for 300 to 800bp).
9. Add 95.8ul of AMPure (x1.8) beads to the supernatant.
10. Repeat steps 2 to 4.
11. Discard the supernatant.
12. Add 200 of 70% EtOH to the beads (for >800bp).
13. Repeat steps 11 and 12.
14. Discard the supernatant.
15. Off the magnet plate, add 40 ul of buffer EB (QIAGEN) to the beads.
16. Pipette 10 times.
17. Incubate on the magnet plate for 1 min.
18. Transfer the supernatant to a new 1.5ml tube.
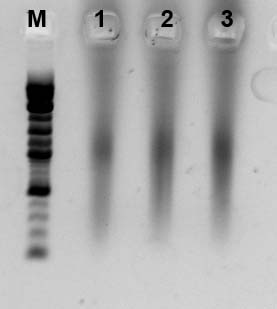
Sample of 300 to 800bp was run out for the next step.
<300bp

>800bp

Confirmation of the library by PCR
| Reaction mixture
|
conc.
|
1x
|
x3.5
|
'
|
lane
|
'
|
| Temp (<300bp, 300to800bp, >800bp) |
|
0.5 |
|
|
M |
NEB 2log marker
|
| TopTaq Buffer |
10x |
1 |
3.5 |
|
1 |
<300 bp
|
| dNTP mix |
10 mM each |
0.2 |
0.7 |
|
2 |
300bp to 800bp
|
| Primer mix (454Primer A and B) |
5 uM each |
1 |
3.5 |
|
3 |
>800 bp
|
| TopTaq |
5 U/ul |
0.05 |
0.175 |
|
|
|
| Milli Q |
|
7.25 |
25.375 |
|
|
|
|
|
10 ul |
|
|
|
|
|
|
|
|
|
|
|
| PCR cycle |
|
|
|
|
|
|
| 94C |
3 min |
|
|
|
|
|
|
|
|
|
|
|
|
| 98C |
10 sec |
|
|
|
|
|
| 60C |
1 min |
|
|
|
|
|
| x35 |
|
|
|
|
|
|
|
|
| 72C |
5 min |
|
|
|
|
|
|
 Alien genes
Alien genes





