Mechanobiology and Microscale Devices - Neeraj, Robert Sterian, Eugene Cheong, Luke Boudreau, Bridgit Foss
Introduction
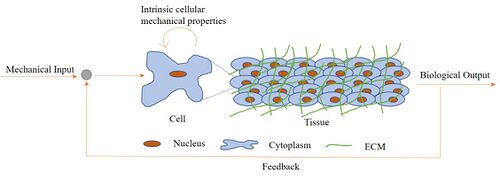
Every organism, be it an amoeba or a complex multicellular organism like human being, is composed of cells which are constantly in communication with the physical environment like the extracellular matrix (ECM), fluid flow, or with adjacent cells. Cells sense their surroundings through biochemical signals like cytokines and growth factors or through mechanical stimuli like osmotic forces, shear stresses, ECM stiffness or geometry.[1] Recently, scientists are beginning to understand that mechanical signals that are either intrinsic or extrinsic play a vital role in cell migration, morphology, proliferation, differentiation and homeostasis.
Mechanotransduction is the process by which a mechanical stimulus elicits a biological response from a cell/tissue which changes the intrinsic properties of a cell or its microenvironment. This in turn modifies the mechanical properties around the cell and creates a feedback loop (Figure 1). Mechanobiology is the field of science which seeks to study the responsiveness and signaling of tissues and cells by altering physical and mechanical properties in order to elucidate the relationships observed in mechanotransduction and cell signaling.[2] For many years, most of the experiments designed to understand the fundamental questions in cell and molecular biology have been performed in vivo with limited control or in vitro in a way that lacks many physiologically relevant parameters. As such microfluidics has become a staple for use in mechanobiology since the micro- and nanoscale aspects of these platforms can allow for a level of control often seen with in vitro experiments, while also providing the robustness of cell differentiation seen with in vivo experiments.[3]
Types of mechanical stimulus
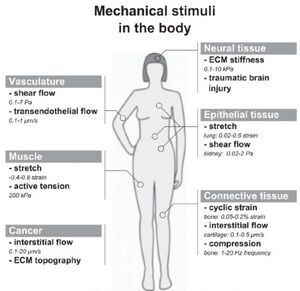
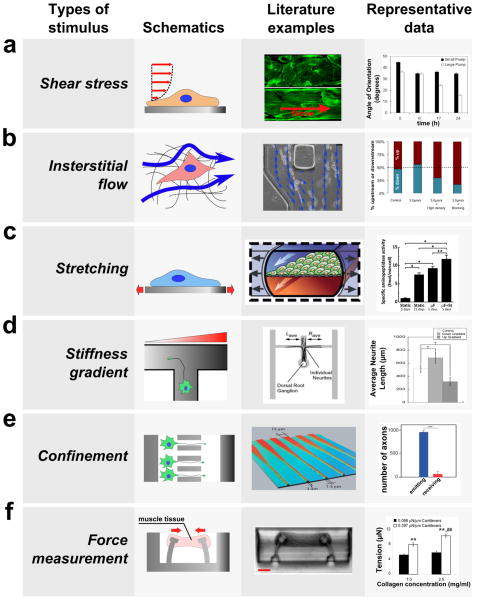
In order to develop more accurate experiments using mechanobiology, microfluidics has been and will be continued to be used to answer mechanobiological questions by developing different microenvironments for cells and tissues to undergo different situations using mechanical devices (Figure 2). The goal in simulating these environments is to gain more knowledge on the interaction of cells and develop more realistic models for in vivo models (Figure 3). The development of realistic models will lead to more efficient methodologies on how to develop experiments with the end goal of achieving organ equilibrium and/or treatment of diseases. The capability of a 3D view of cellular compound interactions between a compound and a surface. For many examples, PDMS is used as a material for many devices. These interactions from different mechanical stimuli will also lead to more accurate observations rather than viewing cells at a 2D plane.[3]
Shear stress in blood vessels
Endothelial shear stress is the friction of blood flow through an endothelial surface.[4] If a blood vessel were to have a high endothelial shear stress, then there is very high friction leading to decrease of blood flow. Where as a blood vessel with a low endothelial shear stress has a low friction leading to an increase of blood flow. With the use of mechanobiology, scientists are able to study the effects of fluid flow by using a constricted pathway to emulate a blood vessel with shear stress. Some responses as a result of this observation are cell elongation, increased cell contractibility, and decreased permeability of blood vessels.[3] Blood vessels are made of endothelial cells (ECs) lining the inner surface, smooth muscle cells and pericytes surrounding the endothelial cell layer and blood cells comprising of erythrocytes, leukocytes and monocytes that travel inside the blood vessel. These cells undergo various mechanical stimuli inside the body, among which the most important component is contributed by hemodynamic forces due to the flow of blood. The ECs experience shear stress on their apical surface generated by the blood flow which influences cell proliferation, morphology, migration, wound healing, permeability and gene expression.
To understand how shear stresses are induced in a blood vessel, let us consider the example of water flowing in a pipe. The flowing water applies a physical force on the walls of the pipe. This force can be resolved into two components: the force parallel to the pipe wall manifests as shear stress and the force perpendicular to the wall exerts a tensile stress. In blood vessels, the shear stress is a frictional force which is exerted by blood on the endothelial side of the vessel whereas the tensile stress is the cause of blood pressure which dilates the vessel wall rhythmically as the heart pumps making it pulsatile in nature.[5][6]
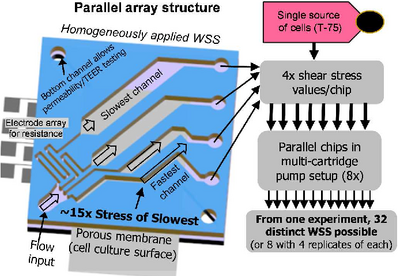
Previous studies involving parallel plate flow chamber models were used to demonstrate the effect of shear flow on ECs but nevertheless these models are difficult to optimize and customize. This is due to the set up requiring a large amount of cells and reagents as it is on a significantly larger scale than its microfluidic counterparts. In addition to this, these plate models also have low experimental efficiency due to its inability to generate multiple shear stress profiles in a single apparatus.[3] In order to eliminate the drawbacks of these macroscale systems, novel microfluidic platforms were used to develop various shear flow assays. Chau et al. have developed a device which allows up to ten different shear stresses spanning across 0.07 – 13 Pa to be induced on the ECs simultaneously (Figure 4).[8] This was achieved by varying the length of the channel, which in turn affected the resistance of the channel (R), volume flow rate (Q) and shear stress (τ).
Transendothelial and interstitial flow in blood vessels
Interstitial flow is defined as the motion of fluids through an extracellular matrix such as tissues. This type of flow is encouraged in order to help transport large proteins and induce physiological responses from interstitial cells.[9] Besides shear flow, the ECs experience transendothelial flow which influences the force and the mass transport across the endothelial layer. These phenomena have been mimicked in vitro using various microfluidic platforms that use 3D collagen hydrogels to study the effect on sprouting angiogenesis (creation of new blood vessels). By implementing a pressure fluid gradient on a hydrogel, motion and alignment of cells can be mimicked as interstitial flow. Song et al. have shown using a microfluidic tissue analog of angiogenic sprouting that fluid shear stress exerted during blood flow reduces EC sprouting in a nitric-oxide dependent manner. Alongside this, it also reduces the interstitial flow witnessed in an extravasating plasma, which directs EC morphogenesis and sprout formation.[3][10]
Strain, stretching, and stiffness in blood vessels
Due to the pulsatile nature of blood flow in the vessels, the ECs, smooth muscle cells (SMCs) and mesenchymal stem cells (MSCs) on blood vessels are exposed to cyclic substrate strain. Various microfluidic assays were developed to study this effect in MSCs and SMCs. Zhou et al. have developed a device to simulate and study the effect of substrate strains when applied on MSCs. The human MSCs (hMSCs) were cultured on flexible membranes which inflates and deflates when pressure is applied and released respectively mimicking the arterial vessels. The study has reported that cells aligned for strains greater than 10% and activated SMAD1/SMAD2 and Wnt/β-catenin pathways.[11]
While strain is often seen in blood vessels, stretching and stiffness is more often observed within muscle cells. Stretching is a form of mechanical stimulation that engineers the physical lining of the membrane and modifies it. This technique is primarily used for promoting the process of myogenesis, the formation of muscular tissue. Stretching can also play a major role in the growth and functionality of muscle.[12] There are multiple methods for how a membrane can be stretched depending on using one more more axels. Studies have shown that muscle tissue under embryonic chick cardiomyocyte and neonatal rat cardiomyocyte culture have been studied for approximately a week in order observe the effects while under mechanical stimulation. The results presented evidence of the muscle tissue becoming more enlarged, improved functionality of contracting, and increased tensile strength.[13][14]
Similar to stretching, stiffness gradient has an influence in myogenesis.[3] Cell stiffness is measured using the elastic modulus of the cell. This can be determined by comparing the curve of force to the indentation within the cell membrane.[15] In addition, with an in vitro model, stiffness has the ability to control a range of cellular functionality such as motility, morphology, and response signaling.[16]
Mechanical load on joints
In vivo, mechanical forces are applied to synovial fluid in knee joints during rest and motion. While at rest, although neighboring cartilage takes on the major role holding against forces due to body weight while standing, this downward pressure does still affect this area. Alongside this, synovial fluid during exercise exerts shear forces on neighboring cells. These shear forces will often result in induced signal transduction through MAPKs, also known as mitogen activated protein kinases, in the affected cells. This MAPK pathway can result in cellular activated in various cell lines.[17] Activation results can vary by cell line, but overall this activation will involve the creation of pro-inflammatory materials by involved cells, leading to cell damage or death on a multicellular scale. In the context of synovial fluid, this is particularly studied concerning osteoarthritis. Here precise microenvironments are created so as to properly replicate this mechanical loading applied to cells in order to observe and determine better treatment and prevention methods for osteoarthritis.[18]
Measurement of cellular traction force using microfluidic devices
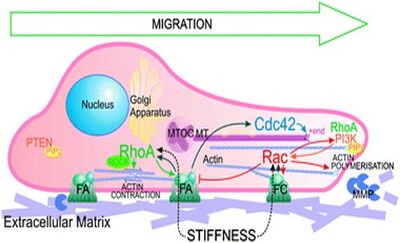
What are cellular traction forces
The cellular traction force (CTF) is defined as the tangential force exerted by cells on the ECM or any underlying substrate. CTFs play a vital role in various biological processes like wound healing, inflammation, cell migration, angiogenesis and metastasis. Generation of CTFs in cells is accomplished using actin filament bundles, also called as stress fibers, and powered by the hydrolysis of ATP generates tension that contracts the cell body due to actomyosin cross bridges. The secondary source of CTF is through actin polymerization which drives the forward protrusion of the leading edge of a migrating cell. These forces are transferred from actin fibers to the ECM through focal adhesions (FA) which consists of a complex assembly of ECM proteins, transmembrane receptors and signaling molecules like integrins, paxillin, vinculin, tensin, talin, kinases and phosphatases. Among the FA proteins, integrins play an important role in cell mechanotransduction by providing a physical connection between the ECM and actin cytoskeleton and this helps in sensing various mechanical cues around the cell microenvironment (Figure 5).[19]
CTFs can be further studied through the use of microfluidic devices. For example, a microfluidic device was developed to study the effect of chemotaxis on migration of a group of cells. The collective movement of cells cultured on PDMS substrates in micropatterned islands were analyzed after a chemical gradient of hepatocyte growth factor (HGF) was induced on said islands. The data was interpreted in terms of monolayer traction and stress microscopy. The PDMS substrate was embedded with fluorescent nanoparticles to track the motion of cells and coated with collagen solution to facilitate cell attachment and proliferation. During the initial time points, all cases showed similar trends where the cells on the edge of the island showed strong inward traction while cells within the island had fluctuations in the traction distribution. After the application of HGF gradient for 10 h, the degree of expansion of the cell island varied but the traction force distributions largely remained the same. However, the monolayer stress maps showed a trend where the stresses were high (200 Pa) when the HGF concentration was low as compared to 100 Pa when the HGF concentration was high.[20]
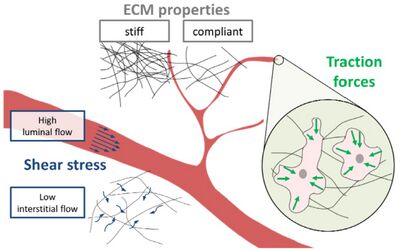
Relevance of CTFs
Traction forces play a vital role in angiogenesis where the coordinated cell movement and ECM remodeling is carried out. The cells migrate in response to complex mechanical and chemical signals around their microenvironment. Mechanical signals like fluid shear stress and ECM stiffness help in blood vessel sprouting and formation (Figure 6). The response of the endothelial cells is communicated in the form of forces exerted by cells on the substrate to remodel them to form appropriate network of blood vessels and capillaries. This forms a feedback loop where mechanosensing of cells through focal adhesions gives them a sense of the mechanical environment around them and in turn they produce traction forces to remodel the matrix to form new blood vessels or repair and maintain existing ones.[21]
Microfluidic devices also provide an alternative, easy and efficient way to compute viscoelastic parameters of cells, binding strengths of biomolecules inside and on the plasma membrane and mechanics of dorsal side of the cell. Atomic force microscopy and optical traps provides a way to compute the molecular binding strengths, traction-force microscopy (TFM) and micropost deflection methods helps in quantifying CTFs and magnetic tweezers help in studying the viscoelastic parameters of the cell.[22]
Micropatterning and Cell Studies
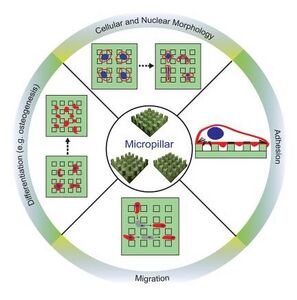
Micropatterning is the creation of a microscopic environment with pre-designed specific topologies that allow the user to control the distribution and spacing of the inserted molecules or cells. There are three types of micropatterns that are regularly used in studies thus far: microgrooves, micropits, and micropillars. These types differ by the structure interacting with the molecules of interest and the pre-set parameters. Microgrooves, for example, are composed of grooves or ridges where ridge depth, width, and spacing between are the main parameters. Micropits on the other hand, are recessed surfaces similar to creating a pit concerning parameters such as pit shape, depth, and width. Finally, micropillars are raised surfaces out of the base layer acting opposite to micropits where once again parameters such as pillar shape, height, and width are relevant but also along with stiffness. [23]
In micropatterning, micropillars are the more favored technique due to their added parameters of stiffness and density allowing the user more opportunities depending on the device purpose. Typically composed of materials such as PDMS or PMMA, micropillar devices are usually fabricated using techniques such as a soft lithography. Micropillars can be used for a variety of applications in mechanobiology when paired with cell culture including observations toward cell changes in morphology, adhesion, and migration patterns (Figure 7). For example, one study in particular used a micropillar device to measure CTFs. The bendability of the pillars due to changing stiffness parameters, allowed for the cells bending around the pillars to be more easily quantifiable, and therefore aiding to calculate the overall forces on the cell. [24]
Similarly, we can quantify CTFs through the deflection of an array of compliant PDMS microposts produced by cells cultured on them. Raman et al. fabricated a microfluidic device consisting of PDMS microposts of varying area. NIH 3T3 and human osteosarcoma cells were used to study the effect of myosin-II and confinement of cells in narrow channels. Inhibition of myosin-II using blebbistatin did not affect the traction force or migration speed of cells in confined narrow channels but did have an effect in wide microchannels whereas activation of myosin-II using calyculin A did not affect the CTF in confined environments but increased them in wide microchannels.[25] This study shows how the physical environment factors can directly affect singular cell process, as the diameter of posts changed CTFs and migration speeds.
Another study surrounding human mesenchymal stem cells (hESCs) utilized other forms of micropatterning through patterns of areas with different degrees of nanoroughness on silica-based glass wafers. This technology was used to observe cell morphology and proliferation as mentioned previously, but also clonal expansion and self-renewal. [26]
Effect of electroporation on cell mechanics
Electroporation (EP) is a common clinical and lab technique where electric pulses are used to transiently permeabilize the cell membrane allowing for rapid delivery of nanoparticles, drugs and genetic material. Generally, cells maintain an electric potential difference between the inner and outer side of the cell membrane due to a complex system of regulation using ion pumps and ion channels in their plasma membrane and this is termed as resting transmembrane voltage (TMV). In eukaryotes, the resting TMV ranges between -40 and -70 mV, i.e. the electric potential inside the cell is lower than the outer one.[28] When an external electric field is applied, it manifests as an additional induced TMV on the cell proportional to the external field besides the resting TMV. This induced voltage is experienced by the cell as long as the duration of the applied field. When the induced TMV exceeds a certain range, then the cell starts to undergo certain structural and molecular changes in its plasma membrane that are absent under normal conditions. One of the changes witnessed due to applied electric field is formation of nanopores on the plasma membrane termed as electropermeabilization which rapidly increases the membrane permeability.[29]
Besides membrane permeabilization, EP induces changes in the actin cytoskeleton and thus affects other mechanical properties of the cell which are essential for its function. The mechanical properties of a cell play a central role in a variety of biological functions such as cell aging, differentiation, migration, mechanotransduction and cell death. These properties can be exploited to serve as a reliable alternative to conventional biomarkers for disease diagnosis. EP can also be used to treat tumors where in the mechanical properties can be changed in order to induce cell death.[30][31]
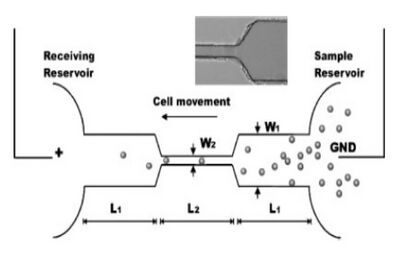
Some studies exist surrounding the effect of EP on cell swelling using a novel high throughput microfluidic device. In this device, EP was performed on a large cell population but on a single cell at a time. CHO cells were allowed to travel through a narrow microchannel where electric field (DC) is greater than the rest of the channel that can cause EP due to change in the length and cross-sectional area for a constant time which can be adjusted based on the velocity of the cells in the fluid. Using the device, Wang et al. were able to capture images and study the changes in cellular morphology and swelling for up to 30 cells per second.[27]
Separately, Bai et al. have used a novel microfluidic approach to characterize the mechanical properties of a cell using dielectrophoresis (DEP) based cell stretching and actin cytoskeleton modeling method (Figure 8). DEP is similar to EP, only instead DEP uses a non-uniform electric field whereas EP uses a uniform electric field. Leukemia cells were treated with a chemotherapeutic drug – doxorubicin that induces apoptosis and altered the actin cytoskeleton. Post treatment, the cells were stretched using two different methods - the DEP setup and optical tweezers to compare the biophysical properties in each case.[32]
Pressure effects on cells
Every cell in the human body experiences some form of pressure. From the bone cells, which experience immense weight to keep our bodies upright, to the blood and aortic cells, which experience large amounts of hydrostatic pressure changes every time out heart beats, pressure is an important variable when studying cells and cell culture, though it is often overlooked. In the field of mechanobiology, researchers aim to study the effect of pressure on cells. Studies that aim to use pressure as a controlled variable must be completed in large and expensive bioreactors that are able to modulate pressure of the system and ensure equal pressure distribution across the cell cultures. Though there is still much to learn about the interactions of cells with pressure, some of the most well studied areas pertain to pressure effects on cellular morphology, mass transport cellular systems, and cellular growth rate.
In regard to cellular morphology, cells will often attempt to compensate immense pressure by increasing pressure internally to counteract the force. Should the cell not compensate, the cell will collapse under the pressure and die. However, if cells are able to balance the pressure with its environment, cells will adopt an elongated morphology (Figure 9).[33]
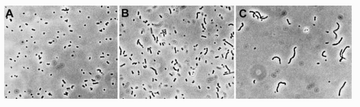
As immense pressure changes the shape of the cell, the cell's membrane and its components are also likely to be affected, though researchers are likely to overlook this. For example, the protein channels within the cellular membrane such as the aquaporin, which allow for the flow of water into and out of the cell, could become deformed and alter rates of diffusion. Alternatively, ion channels could be affected and a neural cell's ability to transmit signals or even synthesize ATP may be affected.[34] Microfluidics may present a novel lens to study pressure on cells. While large bioreactors are extremely viable for pressurizing cell cultures over a large range of pressures, microfluidic devices may be able to bring a similar if not better systems using less resources and space.[35]
One example of this comes from Liu et al. where they created a microfluidic device that has a built-in electrofluidic pressure sensor to study endothelial cells under shear stress as well as hydrostatic pressure.[37] Ho et al. additionally created a microfluidic device that exerts a cyclic hydrostatic pressure on the cells. The system allows for the study of cyclic compression on adherent cells, mimicking the environment experienced by cells in blood vessels.[38]
Non-human applications
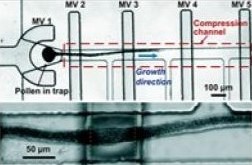
Although the majority of mechanobiology studies in microfluidics surround the use of human or generally animal cells, it has also drawn the attention of non-animal cell applications as well. In these studies, similar factors and methods can be applied but within a different context. Instead of medicinal or drug concerns, plant or generally non-animal sample studies look towards maintaining and further observing environmental health. An example of this is a study surrounding pollen tubes and mechanobiology. Pollen tubes are cylindrical structures found on male plants used to provide sperm cells to other neighboring female plants in order to reproduce. These tubes can be found growing through the pistils of many plant types. However, a directional trigger (a force given a specific direction acting on the pollen tube surface) is necessary to avoid growing in arbitrary directions, hindering its chances to contribute to pollination. These directional triggers are difficult to recreate using current convention methods. This problem lead to the creation of the TipChip (Figure 10). The TipChip is used to position pollen grain with laminar flow to avoid the above mentioned arbitrary growth. The TipChip is also used to study pollen tube reactions when presented with mechanical obstacles, similar to what it would experience in a plant pistil.[36]
References
- ↑ Petridou, N., Spiró, Z. & Heisenberg, CP. Multiscale force sensing in development. Nat Cell Biol 19, 581–588 (2017). https://doi.org/10.1038/ncb3524
- ↑ Shivashankar, G. V.; Sheetz, M.; Matsudaira, P. Mechanobiology. Integr. Biol. 2015, 7 (10), 1091–1092. https://doi.org/10.1039/C5IB90040A.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 Polacheck, W. J.; Li, R.; Uzel, S. G. M.; Kamm, R. D. Microfluidic Platforms for Mechanobiology. Lab Chip 2013, 13 (12), 2252. https://doi.org/10.1039/c3lc41393d.
- ↑ Chatzizisis, Y. S.; Coskun, A.; Jonas, M.; Edelman, E.; Feldman, C.; Stone, P. “Role of Endothelial Shear Stress in the Natural History of Coronary Atherosclerosis and Vascular Remodeling: Molecular, Cellular, and Vascular Behavior.” Journal of the American College of Cardiology, Elsevier, 8 June 2007, https://doi.org/10.1016/j.jacc.2007.02.059
- ↑ White, C. R.; Frangos, J. A. The Shear Stress of It All: The Cell Membrane and Mechanochemical Transduction. Phil. Trans. R. Soc. B 2007, 362 (1484), 1459–1467. https://doi.org/10.1098/rstb.2007.2128.
- ↑ Ulfhammer, E. Impairment of Endothelial Thromboprotective Function by Haemodynamic and Inflammatory Stress Implications for Hypertensive Disease; 2007. https://gupea.ub.gu.se/bitstream/handle/2077/4661/Thesis%20Erik%20Ulfhammer.pdf?sequence=1&isAllowed=y (accessed 2024-04-14).
- ↑ Booth, R., Noh, S., & Kim, H. (2014). A multiple-channel, multiple-assay platform for characterization of full-range shear stress effects on vascular endothelial cells. Lab on a chip, 14 11, 1880-90 . https://doi.org/10.1039/c3lc51304a
- ↑ Chau, L.; Doran, M.; Cooper-White, J. A Novel Multishear Microdevice for Studying Cell Mechanics. Lab Chip 2009, 9 (13), 1897. https://doi.org/10.1039/b823180j.
- ↑ Yao, W.; Li, Y.; Ding, G. Interstitial Fluid Flow: The Mechanical Environment of Cells and Foundation of Meridians. Evidence-Based Complementary and Alternative Medicine 2012, 2012, 1–9. https://doi.org/10.1155/2012/853516.
- ↑ Song, J. W.; Munn, L. L. Fluid Forces Control Endothelial Sprouting. Proc. Natl. Acad. Sci. U.S.A. 2011, 108 (37), 15342–15347. https://doi.org/10.1073/pnas.1105316108.
- ↑ Zhou, J.; Niklason, L. E. Microfluidic Artificial “Vessels” for Dynamic Mechanical Stimulation of Mesenchymal Stem Cells. Integrative Biology 2012, 4 (12), 1487–1497. https://doi.org/10.1039/c2ib00171c.
- ↑ Rangarajan, S.; Madden, L.; Bursac, N. Use of Flow, Electrical, and Mechanical Stimulation to Promote Engineering of Striated Muscles. Ann Biomed Eng 2014, 42 (7), 1391–1405. https://doi.org/10.1007/s10439-013-0966-4.
- ↑ Fink, C.; Ergün, S.; Kralisch, D.; Remmers, U.; Weil, J.; Eschenhagen, T. Chronic Stretch of Engineered Heart Tissue Induces Hypertrophy and Functional Improvement. FASEB j. 2000, 14 (5), 669–679. https://doi.org/10.1096/fasebj.14.5.669.
- ↑ Boublik, J.; Park, H.; Radisic, M.; Tognana, E.; Chen, F.; Pei, M.; Vunjak-Novakovic, G.; Freed, L. E. Mechanical Properties and Remodeling of Hybrid Cardiac Constructs Made from Heart Cells, Fibrin, and Biodegradable, Elastomeric Knitted Fabric. Tissue Engineering 2005, 11 (7–8), 1122–1132. https://doi.org/10.1089/ten.2005.11.1122.
- ↑ (1)Luo, Q.; Kuang, D.; Zhang, B.; Song, G. Cell Stiffness Determined by Atomic Force Microscopy and Its Correlation with Cell Motility. Biochimica et Biophysica Acta (BBA) - General Subjects 2016, 1860 (9), 1953–1960. https://doi.org/10.1016/j.bbagen.2016.06.010.
- ↑ Isenberg, B. C.; DiMilla, P. A.; Walker, M.; Kim, S.; Wong, J. Y. Vascular Smooth Muscle Cell Durotaxis Depends on Substrate Stiffness Gradient Strength. Biophysical Journal 2009, 97 (5), 1313–1322. https://doi.org/10.1016/j.bpj.2009.06.021.
- ↑ Schett G, Tohidast-Akrad M, Steiner G, Smolen J. The stressed synovium. Arthritis Res. 2001;3(2):80-86. https://doi:10.1186/ar144
- ↑ Zou Z, Luo X, Chen Z, Zhang YS, Wen C. Emerging microfluidics-enabled platforms for osteoarthritis management: from benchtop to bedside. Theranostics. 2022;12(2):891-909. Published 2022 Jan 1. https://doi:10.7150/thno.62685
- ↑ 19.0 19.1 Wang, J. H.; Lin, J. S. (2007). Cell traction force and measurement methods. Biomechanics and modeling in mechanobiology, 6(6), 361–371. https://doi.org/10.1007/s10237-006-0068-4
- ↑ Jang, H.; Kim, J.; Shin, J. H.; Fredberg, J. J.; Park, C. Y.; Park, Y. Traction Microscopy Integrated with Microfluidics for Chemotactic Collective Migration. JoVE (Journal of Visualized Experiments) 2019, No. 152, e60415. https://doi.org/10.3791/60415.
- ↑ 21.0 21.1 Boldock, L., Wittkowske, C., & Perrault, C. M. (2017). Microfluidic traction force microscopy to study mechanotransduction in angiogenesis. Microcirculation (New York, N.Y. : 1994), 24(5), 10.1111/micc.12361. https://doi.org/10.1111/micc.12361
- ↑ Park, S., Joo, Y.K. & Chen, Y. Versatile and High-throughput Force Measurement Platform for Dorsal Cell Mechanics. Sci Rep 9, 13286 (2019). https://doi.org/10.1038/s41598-019-49592-1
- ↑ 23.0 23.1 Long, Y., Sun, Y., Jin, L., Qin, Y., & Zeng, Y. (2023). Micropillars in biomechanics: Role in guiding mesenchymal stem cells differentiation and bone regeneration. Advanced Materials Interfaces, 11(2). https://doi.org/10.1002/admi.202300703
- ↑ Amer, M., Wolfenson, H. (2023). Measuring Cellular Traction Forces with Micropillar Arrays. In: Zaidel-Bar, R. (eds) Mechanobiology. Methods in Molecular Biology, vol 2600. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2851-5_13
- ↑ Raman, P. S., Paul, C. D., Stroka, K. M., & Konstantopoulos, K. (2013). Probing CTFs in confined microenvironments. Lab on a Chip, 13(23), 4599-4607. https://doi.org/10.1039/c3lc50802a
- ↑ Chen, W., Villa-Diaz, L. G., Sun, Y., Weng, S., Kim, J. K., Lam, R. H., Han, L., Fan, R., Krebsbach, P. H., & Fu, J. (2012). Nanotopography influences adhesion, spreading, and self-renewal of human embryonic stem cells. ACS Nano, 6(5), 4094–4103. https://doi.org/10.1021/nn3004923
- ↑ 27.0 27.1 Wang, H.-Y.; Lu, C. High-Throughput and Real-Time Study of Single Cell Electroporation Using Microfluidics: Effects of Medium Osmolarity. Biotechnol. Bioeng. 2006, 95 (6), 1116–1125. https://doi.org/10.1002/bit.21066.
- ↑ Kotnik, T.; Rems, L.; Tarek, M.; Miklavčič, D. Membrane Electroporation and Electropermeabilization: Mechanisms and Models. Annual Review of Biophysics 2019, 48 (1), 63–91. https://doi.org/10.1146/annurev-biophys-052118-115451.
- ↑ S. M. Kennedy, Z. Ji, J. C. Hedstrom, J. H. Booske, and S. C. Hagness, “Quantification of electroporative uptake kinetics and electric field heterogeneity effects in cells,” Biophys. J., vol. 94, no. 12, pp. 5018–5027, 2008. https://doi.org/10.1529/biophysj.106.103218
- ↑ Kodama, H.; Vroomen, L. G.; Eisuke Ueshima; Reilly, J.; Brandt, W.; Paluch, L.-R.; Monette, S.; Jones, D.; Solomon, S. B.; Govindarajan Srimathveeravalli. Catheter-Based Endobronchial Electroporation Is Feasible for the Focal Treatment of Peribronchial Tumors. Journal of thoracic and cardiovascular surgery (Print) 2018, 155 (5), 2150-2159.e3. https://doi.org/10.1016/j.jtcvs.2017.11.097.
- ↑ Kodama, H.; Shamay, Y.; Kimura, Y.; Shah, J.; Solomon, S. B.; Heller, D.; Srimathveeravalli, G. Electroporation-Induced Changes in Tumor Vasculature and Microenvironment Can Promote the Delivery and Increase the Efficacy of Sorafenib Nanoparticles. Bioelectrochemistry (Amsterdam, Netherlands) 2019, 130, 107328. https://doi.org/10.1016/j.bioelechem.2019.107328.
- ↑ Bai, G., Li, Y., Chu, H.K. et al. Characterization of biomechanical properties of cells through dielectrophoresis-based cell stretching and actin cytoskeleton modeling. BioMed Eng OnLine 16, 41 (2017). https://doi.org/10.1186/s12938-017-0329-8
- ↑ 33.0 33.1 Molina-Höppner, A.; Sato, T.; Kato, C.; Gänzle, M. G.; Vogel, R. F. Effects of Pressure on Cell Morphology and Cell Division of Lactic Acid Bacteria. Extremophiles 2003, 7 (6), 511–516. https://doi.org/10.1007/s00792-003-0349-0.
- ↑ The Effects of Pressure on the Molecular Structure and Physiological Functions of Cell Membranes. 2022, 23.
- ↑ Tworkoski, E.; Glucksberg, M. R.; Johnson, M. The Effect of the Rate of Hydrostatic Pressure Depressurization on Cells in Culture. PLoS ONE 2018, 13 (1), e0189890. https://doi.org/10.1371/journal.pone.0189890.
- ↑ 36.0 36.1 Yanagisawa, N.; Kozgunova, E.; Grossmann, G.; Geitmann, A.; Tetsuya Higashiyama. Microfluidics-Based Bioassays and Imaging of Plant Cells. Plant & cell physiology/Plant and cell physiology 2021, 62 (8), 1239–1250. https://doi.org/10.1093/pcp/pcab067.
- ↑ Liu, M.-C.; Shih, H.-C.; Wu, J.-G.; Weng, T.-W.; Wu, C.-Y.; Lu, J.-C.; Tung, Y.-C. Electrofluidic Pressure Sensor Embedded Microfluidic Device: A Study of Endothelial Cells under Hydrostatic Pressure and Shear Stress Combinations. Lab Chip 2013, 13 (9), 1743. https://doi.org/10.1039/c3lc41414k.
- ↑ Ho, K. K. Y.; Wang, Y. L.; Wu, J.; Liu, A. P. Advanced Microfluidic Device Designed for Cyclic Compression of Single Adherent Cells. Front. Bioeng. Biotechnol. 2018, 6, 148. https://doi.org/10.3389/fbioe.2018.00148.
