Etchevers:Notebook/Genomics of hNCC/2008/11/25
 Genetics of human eye development Genetics of human eye development
|
|
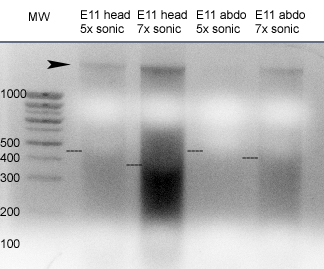
Results from sonication trials on E11 mouse tissuesSince I set up a redirect to this entry, here is the photo of the sonication. To go on paging forward in the transcription factors in eye development project, click here and to move back to the last entry, here. The majority of fragments migrate below 450 bp for the 5x pulses and below 300 for the 7x pulses, so will do 4x pulses for next try with the 10 eyes. The arrowhead is, I believe, unfragmented or poorly fragmented, large DNA. It at least moves out of the well. They also get some filamentous stuff rising from the pellet, that sounds just like long chromatin to me. I suggested putting less tissue. E11 = Theiler 18 = C15 so that's just perfect. The estimations are as follows:
Showing CMFDA distribution in rabbit corneal sectionsLast September 26th, we injected hNCC from either cephalic or trunk levels into the injured rabbit corneas of four rabbits. Angelique finished sectioning about 20 4-7 μm sections into each middle area of the cornea (they had been cut in half more or less and cut edges embedded down). We are using the recently received anti-fluorescein-R-phycoerythrin to see if can convert old exhausted fluorescein into bright red fluorescence. 1. Deparaffinated sections (did this before I gave her my more progressive protocol): 10' at 60 °C and melted paraffin 2x 4’ xylene (or toluene or Histoclear) 2x 4’ 100% EtOH 5' in running water Passage in PBS 2. Glycine (0.15% w/v in PBS) 10 min. 3. Passage PBS. 4. Block non-specific antibody binding with 10% goat serum in PBS for 30’. 300 μL per slide without a coverslip, this is preferable but be careful about drying. 5. Can reduce background as well by using Image-it Fx (Invitrogen I36933): apply a couple drops for 30’ under Parafilm coverslip. 6. Rinse PBS. 7. Primary antibody dilution 1:200 in 10% serum/PBS (anti-fluo-RPE or IgG) • Place 100μL per slide under glass/plastic coverslip in humid chamber. • Incubate 2 hours at room temperature (RT). 8. Rinse 5x PBS for 5’ each (at least). 9. Drain to nearly dry, and mount with ProLong Gold from Invitrogen/Molecular Probes with DAPI already in it (P-36931): warm to room temperature just before use and keep dessicated. 10. Leave to cure at 4ºC o/n then nail polish around coverslips.
Negotiated a new sample of RAX antibody (cf. this entry ) with Millipore and aliquotted some more. Embryo embedding
| |