User:Mary Mendoza/Notebook/CHEM572 Exp. Biological Chemistry II/2013/02/20: Difference between revisions
From OpenWetWare
Mary Mendoza (talk | contribs) (Autocreate 2013/02/20 Entry for User:Mary_Mendoza/Notebook/CHEM572_Exp._Biological_Chemistry_II) |
Mary Mendoza (talk | contribs) |
||
| (15 intermediate revisions by the same user not shown) | |||
| Line 6: | Line 6: | ||
| colspan="2"| | | colspan="2"| | ||
<!-- ##### DO NOT edit above this line unless you know what you are doing. ##### --> | <!-- ##### DO NOT edit above this line unless you know what you are doing. ##### --> | ||
== | ==Aspirin concentration for ADA Kinetic Assay== | ||
* | * The assay for the concentrations below will be conducted next laboratory period. | ||
[[Image:Screen_Shot_2013-02-19_at_1.13.00_PM.png|center]] | |||
==ADA Kinetic Assay for obtaining the zero point== | |||
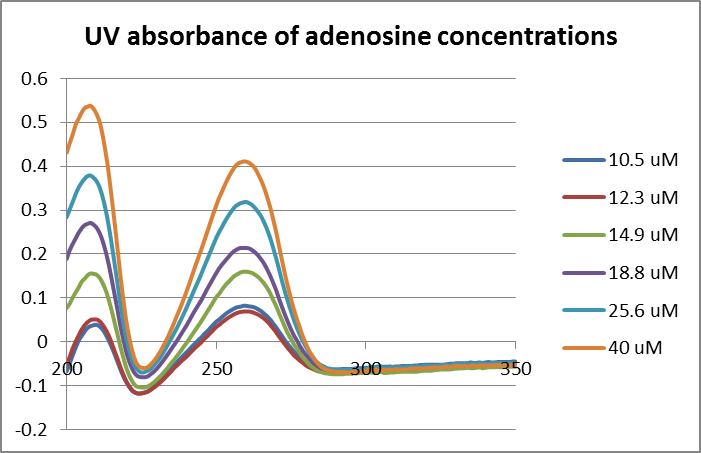
* The objective of this laboratory period is to conduct adenosine deaminase (ADA) kinetic assay runs for the new calculated concentrations. | |||
* UV 2550 Shimadzu spectrophotometer was baseline with 0.05 M sodium phosphate buffer. | |||
* The assays were prepared according to the data below. | |||
[[Image:Screen_Shot_2013-02-19_at_2.46.05_PM.png|center]] | |||
* After running the first trial, it was observed that the absorbance for 12.34 μM adenosine of trial 2 was superimposed over the 10.52 μM adenosine of trial 1. | |||
* It was suggested by Dr. Hartings to use Beer's Law for accurate measurement of the concentration of adenosine in solution. | |||
* An article was provided by Dr. Hartings with the molar extinction coefficient, 1.53 x 10<sup>-4</sup> of adenosine at 260 nm. | |||
* Thus, it was decided to run a full spectrum of adenosine at each given concentration specified above for accurate measurement of the concentration of adenosine. | |||
==Data== | |||
[[Image:Concen1.png|center]] | |||
[[Image:AveVelo1.png|center]] | |||
[[Image:1adeno.png|center]] | |||
[[Image:Lin1.png|center]] | |||
[[Image:UV2.20.png|center]] | |||
<!-- ##### DO NOT edit below this line unless you know what you are doing. ##### --> | <!-- ##### DO NOT edit below this line unless you know what you are doing. ##### --> | ||
Revision as of 12:29, 22 February 2013
| <html><img src="/images/9/94/Report.png" border="0" /></html> Main project page <html><img src="/images/c/c3/Resultset_previous.png" border="0" /></html>Previous entry<html> </html>Next entry<html><img src="/images/5/5c/Resultset_next.png" border="0" /></html> | |
Aspirin concentration for ADA Kinetic Assay
 ADA Kinetic Assay for obtaining the zero point

Data



 | |