User:Alexander Cvitan/Notebook/Experimental Biological Chemistry Lab/2014/04/08: Difference between revisions
From OpenWetWare
| Line 38: | Line 38: | ||
==Figures== | ==Figures== | ||
[[Image:4.8.aa.all.png|700px|]] | [[Image:4.8.aa.all.png|700px|]] | ||
*2,2 Bipyridine was shown on a separate graph because the concentrations of variable used are vastly different form the other variables. This was done because availability of this variable was less than the others. | |||
[[Image:4.8.2.2.bipy.png|700px|]] | |||
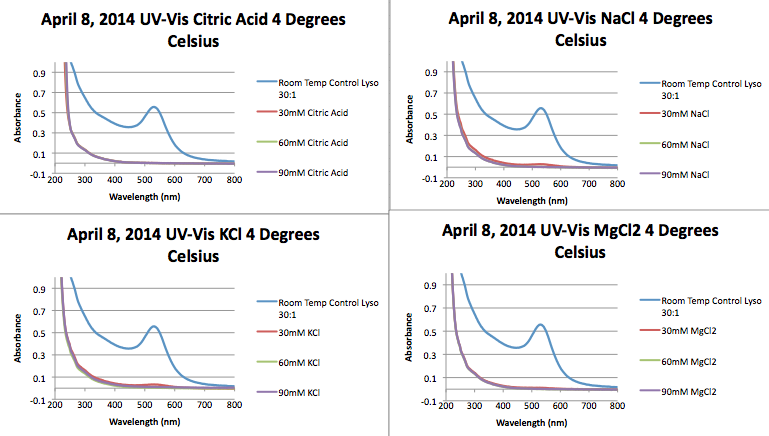
== UV-Vis== | == UV-Vis== | ||
*The following UV-Vis are for Lyso 30:1 samples made on March 26, 2014. The samples were synthesized using the method we've been using all year and variable was added added synthesis. The samples were then cooled to 4 Degrees to see the effect on fiber formation. | *The following UV-Vis are for Lyso 30:1 samples made on March 26, 2014. The samples were synthesized using the method we've been using all year and variable was added added synthesis. The samples were then cooled to 4 Degrees to see the effect on fiber formation. | ||
Revision as of 15:18, 17 April 2014
 Biomaterials Design Lab: Spring 2014 Biomaterials Design Lab: Spring 2014
|
<html><img src="/images/9/94/Report.png" border="0" /></html> Main project page <html><img src="/images/c/c3/Resultset_previous.png" border="0" /></html>Previous entry<html> </html>Next entry<html><img src="/images/5/5c/Resultset_next.png" border="0" /></html> |
Objective
ProcedureConductivity Measurement of Pure Variables at Room Temperature
Atomic Absorption PreparationCreating the Gold Stock Solutions
Atomic Absorption Samples Solutions with the following Au:lysozyme ratio at 4°C were run on the AA:
Figures
UV-Vis
| |