Tamoxifen: Difference between revisions
(chemical data moved from tam administration) |
(table reworked) |
||
| Line 3: | Line 3: | ||
Tamoxifen is a common breast cancer drug (Nolvadex, Istubal, and Valodex). In molecular biology tamoxifen activates enzymes inactivated by fusion to a modified estrogen receptor (ER). This is employed, for example, in the timed activation of Cre recombinase in CreER mice. | Tamoxifen is a common breast cancer drug (Nolvadex, Istubal, and Valodex). In molecular biology tamoxifen activates enzymes inactivated by fusion to a modified estrogen receptor (ER). This is employed, for example, in the timed activation of Cre recombinase in CreER mice. | ||
== Chemical data for | == Chemical data for tamoxifen == | ||
{| {{table}} | |||
{ | |||
|- | |- | ||
| tamoxifen forumlation | |||
| molecular weight | | molecular weight | ||
| pKa' | | pKa' | ||
| equilibrium solubility in water at 37°C | | equilibrium solubility in water at 37°C | ||
| see also | |||
|- | |- | ||
| | | tamoxifen | ||
| | | xx g/mol | ||
| | | xx | ||
| | | xx mg/mL | ||
| [http://www.rxlist.com/cgi/generic/tamox.htm RX] | |||
|- | |- | ||
| 4-hydroxytamoxifen (OHT) | |||
| 387.51 g/mol | | 387.51 g/mol | ||
| ? | | ? | ||
| ? mg/mL | | ? mg/mL | ||
| [http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=449459 PubChem] | |||
|- | |||
| tamoxifen citrate | |||
| 563.62 g/mol | |||
| 8.85 | |||
| 0.5 mg/mL | |||
| [http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=2733525 PubChem] | |||
|} | |} | ||
== Molecular mechanism of action == | == Molecular mechanism of action == | ||
[[Image:Tamoxifen(OH).gif|right|thumb|150px|chemical structure of tamoxifen and 4-hydroxy-tamoxifen]] | |||
Tamoxifen competitively binds to GPCR type and intracellular type estrogen receptors (ER). Binding to the intracellular ER on tumours and other tissue targets produces a nuclear complex that decreases DNA synthesis and inhibits estrogen effects ([http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=endocrin&part=A972&rendertype=box&id=A1246 diagram of estrogen & tamoxifen mechanism]). It thus acts as an estrogen antagonists which inhibits the growth of some tumours dependent on estrogen stimulation. | Tamoxifen competitively binds to GPCR type and intracellular type estrogen receptors (ER). Binding to the intracellular ER on tumours and other tissue targets produces a nuclear complex that decreases DNA synthesis and inhibits estrogen effects ([http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=endocrin&part=A972&rendertype=box&id=A1246 diagram of estrogen & tamoxifen mechanism]). It thus acts as an estrogen antagonists which inhibits the growth of some tumours dependent on estrogen stimulation. | ||
Tamoxifen itself is a prodrug, having relatively little affinity for the estrogen receptor. It is metabolised in the liver by the cytochrome P450 to active metabolites such as 4-hydroxytamoxifen and N-desmethyl-4-hydroxytamoxifen. | Tamoxifen itself is a prodrug, having relatively little affinity for the estrogen receptor. It is metabolised in the liver by the cytochrome P450 to active metabolites such as 4-hydroxytamoxifen (see figure) and N-desmethyl-4-hydroxytamoxifen. | ||
== Activation of ER-fusion proteins == | == Activation of ER-fusion proteins == | ||
Revision as of 05:46, 7 April 2009
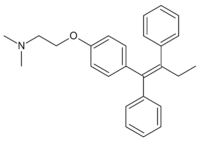
Tamoxifen is a common breast cancer drug (Nolvadex, Istubal, and Valodex). In molecular biology tamoxifen activates enzymes inactivated by fusion to a modified estrogen receptor (ER). This is employed, for example, in the timed activation of Cre recombinase in CreER mice.
Chemical data for tamoxifen
| tamoxifen forumlation | molecular weight | pKa' | equilibrium solubility in water at 37°C | see also |
| tamoxifen | xx g/mol | xx | xx mg/mL | RX |
| 4-hydroxytamoxifen (OHT) | 387.51 g/mol | ? | ? mg/mL | PubChem |
| tamoxifen citrate | 563.62 g/mol | 8.85 | 0.5 mg/mL | PubChem |
Molecular mechanism of action

Tamoxifen competitively binds to GPCR type and intracellular type estrogen receptors (ER). Binding to the intracellular ER on tumours and other tissue targets produces a nuclear complex that decreases DNA synthesis and inhibits estrogen effects (diagram of estrogen & tamoxifen mechanism). It thus acts as an estrogen antagonists which inhibits the growth of some tumours dependent on estrogen stimulation.
Tamoxifen itself is a prodrug, having relatively little affinity for the estrogen receptor. It is metabolised in the liver by the cytochrome P450 to active metabolites such as 4-hydroxytamoxifen (see figure) and N-desmethyl-4-hydroxytamoxifen.
Activation of ER-fusion proteins
Proteins fused to an estrogen receptor (ER) domain are sequestered by heat shock proteins (hsp). Administration of tamoxifen to an often modified ER with increased affinity for tamoxifen over the natural substrate estrogen, releases the fusion protein. In the case of an enzyme-ER fusion, like CreER, it can than act on its substrate.
Stability & storage
Chemical stability (tamoxifen citrate [1])
- stable for at least 2 years when stored desiccated at 2-8°C in the dark
- hygroscopic at high relative humidities
- sensitive to UV light
Metabolism
- distribution half-life: 7-14 hours
- elimination half-life: 5-7 days (range: 3-21 days)
- elimination half-life of N-desmethyltamoxifen (major metabolite): 9-14 days
(estimates based on limited data probably from humans [2])