OpenSourceMalaria:GSK Arylpyrrole Series: Difference between revisions
Matthew Todd (talk | contribs) (→The Arylpyrrole Series: Tweaked start of page) |
Matthew Todd (talk | contribs) (→Other known incidences of these molecules/this series: added info about ubiquitin inhibitor) |
||
| Line 99: | Line 99: | ||
=== Other known incidences of these molecules/this series === | === Other known incidences of these molecules/this series === | ||
Related compounds are known to inhibit the proteasome, according to [http://www.nature.com/nature/journal/v467/n7312/abs/nature09299.html this Nature paper]. | Related compounds are known to inhibit the proteasome, according to [http://www.nature.com/nature/journal/v467/n7312/abs/nature09299.html this Nature paper]. There is a related set of slides from a company, Progenra. The lead compound (IU1, CAS 314245-33-5) is [http://www.sigmaaldrich.com/catalog/product/sigma/i1911?lang=en®ion=AU commercially available]. | ||
According to [http://dx.doi.org/10.1016/j.bmcl.2011.09.049 this paper], similar compounds are agonists of the sphingosine-1-phosphate receptor subtypes 1-5, which have a role in immune system function. | According to [http://dx.doi.org/10.1016/j.bmcl.2011.09.049 this paper], similar compounds are agonists of the sphingosine-1-phosphate receptor subtypes 1-5, which have a role in immune system function. | ||
Revision as of 05:19, 12 July 2013
The Arylpyrrole Series
This page contains a summary of all the data associated with arylpyrrole series ("Series 1") of the Open Source Malaria project. A more human-readable summary is available. For higher-level information, see the OSM Landing Page
The two subseries have the general structure:
- TCMDC Aryl pyrrole core InChi Key: LNROIXNEIZSESG-UHFFFAOYSA-N
- Near-Neighbour core InChi Key: JZQOEGKHCXONAV-ZROIWOOFSA-N


A number of these compounds and intermediates are available for biological testing from by request (contact information on the Landing Page). If you would like to contribute new analogs, please check the list of desired compounds.
The Two Known TCMDC Series Starting Points
Compounds are commercially-available
Series was released in a large dataset by GSK and later listed as one of the most promising leads from TCAMS set (though lacking aryl F)
TCMDC 123812

InChI=1/C15H15FN2O3/c1-9-7-13(15(20)21-8-14(17)19)10(2)18(9)12-5-3-11(16)4-6-12/h3-7H,8H2,1-2H3,(H2,17,19)
SMILES: O=C(N)COC(=O)c2c(n(c1ccc(F)cc1)c(c2)C)C
CAS Registry Number: 733026-12-5
Chemspider page
ChEMBL Page
Resynthesis:
TCMDC 123794

InChI=1/C26H25FN4O4/c1-16-14-22(17(2)30(16)20-12-10-19(27)11-13-20)26(34)35-15-23(32)28-24-18(3)29(4)31(25(24)33)21-8-6-5-7-9-21/h5-14H,15H2,1-4H3,(H,28,32)
SMILES: Fc1ccc(cc1)n2c(cc(c2C)C(=O)OCC(=O)NC=4C(=O)N(c3ccccc3)N(C=4C)C)C
Chemspider page
ChEMBL Page
Resynthesis:
The proposed resynthesis strategy for these two compounds:

The Near-Neighbour Sub-series
Known "Near Neighbours" contained in the Tres Cantos set
The original idea for investigation of the near-neighbour set came from Paul Willis and was posted on the synaptic leap.

Data/links for these compounds:
TCMDC-123563, CHEMBL546966, CHEMBL page: 637010 Cc1ccc(cc1)n2c(cc(c2C)C(=O)CN3C(=O)C(NC3=O)Cc4ccccc4)C
TCMDC-125698, CHEMBL587989, CHEMBL: 627784 Cc1cc(c(n1c2ccc(cc2)Cl)C)C=C3C(=O)N(C(=Nc4ccccc4)S3)C5CCCC5
TCMDC-125697, CHEMBL581336, CHEMBL: 640978 CCOC(=O)c1ccc(cc1)n2c(cc(c2C)C=C3C(=O)N(C(=Nc4ccccc4)S3)C5CCCC5)C
TCMDC-125659, CHEMBL528140, CHEMBL: 626220 Cc1ccnc(c1)n2c(cc(c2C)C=C3C(=O)N(C(=Nc4ccccc4)S3)Cc5ccco5)C
TCMDC-124103, CHEMBL588465, CHEMBL: 643107 Cc1cc(cc(c1)n2c(cc(c2C)C=C3C(=O)NC(=Nc4ccc(cc4)Cl)S3)C)C
TCMDC-124456, CHEMBL548395, CHEMBL: 640006 CCn1c(cc(c1C)C=C2C(=O)NC(=Nc3ccccc3)S2)C
Initial synthesis strategy toward near-neighbours

Experimental information available from PMY 13-1, PMY 14-1 and PMY 16-1, PMY 14-3.
Current synthesis strategy toward near-neighbours

Synthesis method taken from this paper.
Alternative side-chains
Some alternative heterocyclic side-chain ideas have been proposed here at the Synaptic Leap.
Oxazoles
The [1] side chain would target ester isosteres (see TCMDC-123812) but with greater biological stability.
- Synthesis: oxazole synthesis
- Pyrrole core acid to amide: Amide Synthesis
Other known incidences of these molecules/this series
Related compounds are known to inhibit the proteasome, according to this Nature paper. There is a related set of slides from a company, Progenra. The lead compound (IU1, CAS 314245-33-5) is commercially available.
According to this paper, similar compounds are agonists of the sphingosine-1-phosphate receptor subtypes 1-5, which have a role in immune system function.
A new class of antimalarial was recently published by Novartis, Imidazolopiperazines.
Misc papers to digest/assimilate regarding antimycobacterial/tuberculosis activity: Antimycobacterial 1,5-diphenyl pyrroles, 10.1016/j.ejmech.2009.06.005, 10.1016/j.bmc.2010.09.006, 10.1002/cmdc.201000526
ChEMBL-NTD
Searches on ChEMBL-NTD reveal:
- 19 iminothiazolidinones
- 55 2,5-dialkylpyrroles
- 58 rhodanines. These do not contain any biological data, presumably omitted due to promiscuity/PAINS issues.
Scifinder Search: 2,4,5-alkyl-1-aryl-pyrrole: 77552 hits, 905 biological studies:
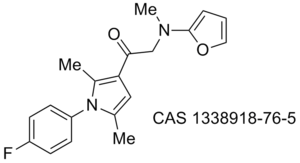
Obesity/diabetes GIP receptor inhibitor JP 2011184298 (A)

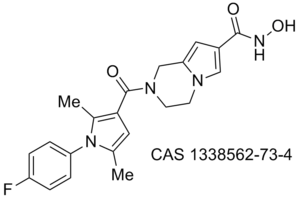
Proteasome WO 2011094545 (A2)

WO2006076202 Steroid nuclear receptor ligands
WO 2011075684 (A1) Inhibitors of Plasma Kallikrein/Protease
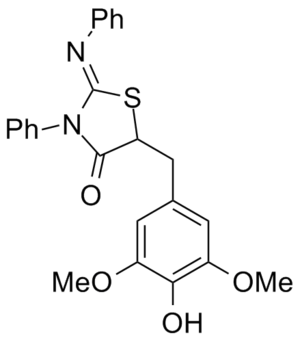
WO 2009137133 (A2) 5-Substituted-2-Imino-Thiazolidinone Compounds And Their Use As Inhibitors Of Bacterial Infection
Synthetic Routes to Analogs Proposed By Others
The CRO Asclepia, via Frederik Doroose, contributed these synthetic routes to the project:
sD and sI compounds: Word file. sC, sD, sF, sJ, sI: Word file. Note prices are for S/M and should not be taken as a quote value.
Biology
Malaria Assays
In vitro
Current tested compounds and their biological activities are summarised on a Google spreadsheet
The results are collected on malaria.ourexperiment.org.
In vivo
TCMDC-123812, TCMDC-123794 and near neighbour ZYH 3-1 have been submitted for study oral in vivo mouse model. Unfortunately it was found that the compounds possess zero oral efficacy (Results available here) in this initial trial.
Mode of action
In silico prediction
In silico prediction of targets has been carried out by Iain Wallace and summarised by Mat on the synaptic leap. Detail on the procedure is given on the ELN. The following targets were identified from data derived from the ChEMBL database:
- Carboxy-terminal domain RNA polymerase II polypeptide A small phosphatase 1
- Dihydroorotate dehydrogenase (DHODH)[in progress at GSK Tres Cantos]
- SUMO-activating enzyme subunit 2
- SUMO-activating enzyme subunit 1
- Cyclin-dependent kinase 1
Collaborators are invited to investigate these targets to confirm if these are targeted by our compounds.
In vitro
GSK Tres Cantos have offered to assay our compounds against dihydroorotate dehydrogenase (DHODH).
