M465:Bootcamp: Difference between revisions
| Line 99: | Line 99: | ||
4. Place the loop with the bacterial growth into the drop of water on the slide. Use a circular motion to make a smooth suspension of the bacteria in the water. Stop when there is a circle of emulsified bacteria about the size of a nickle on the slide.<br> | 4. Place the loop with the bacterial growth into the drop of water on the slide. Use a circular motion to make a smooth suspension of the bacteria in the water. Stop when there is a circle of emulsified bacteria about the size of a nickle on the slide.<br> | ||
5. Reflame the loop.<br> | 5. Reflame the loop.<br> | ||
6. Repeat steps 1-4 with the other cultures. | 6. Repeat steps 1-4 with the other cultures. <br> | ||
7. Allow the slide to air dry completely! Be sure ''all'' the water on the slide has evaporated before proceeding to heat fixation!!! This drying step is crucially important. If you are impatient, you will "explode" the cells in the next step . <br> | 7. Allow the slide to air dry completely! Be sure ''all'' the water on the slide has evaporated before proceeding to heat fixation!!! This drying step is crucially important. If you are impatient, you will "explode" the cells in the next step . <br> | ||
8. Heat fix (to kill and attach organisms to the slide) by quickly passing the slide (smear side up) through a flame 3 times. Your instructors will provide ethanol lamps or bunsen burners to use in the hoods for this purpose. Use a clothes pin or slide holder and avoid contact with hot glass.<BR><BR> | 8. Heat fix (to kill and attach organisms to the slide) by quickly passing the slide (smear side up) through a flame 3 times. Your instructors will provide ethanol lamps or bunsen burners to use in the hoods for this purpose. Use a clothes pin or slide holder and avoid contact with hot glass.<BR><BR> | ||
Revision as of 11:27, 27 January 2013
Microbiology Boot Camp
In this first lab you will learn:
- Who microbiologists are and what they do
- Aseptic technique: don't contaminate yourself or your cultures!
- The basic equipment and procedures used in microbiological investigation
- Using a lab notebook to record the progress of your experiments
Introduction To Microbiology
Welcome to the unseen world of microorganisms. For most of us, microbes are out of sight and out of mind; largely, the human population would prefer it that way. However, since microbes have a major and continuing impact on us and on our planet, it behooves us to understand them better. By the end of this course, you will. Understanding the microbial world is a huge undertaking. A discipline that defines its scope as including all life forms (and some non-life forms like viruses and prions) that are invisible to the unaided human eye is a bit like saying we will study all humans and other animals shorter than 4 ft. Besides including a huge number of members, the diversity of such a group is overwhelming.
So where do we begin in the study of microbiology? It's good to start with appreciating the power of these tiny, unseen life forms to thrive and spread without our permission or knowledge. It is also wise to recognize that although a tiny fraction of the microbes in our world are disease causing, there are devastating infections caused by microbial pathogens. Although none of the microorganisms that we will knowingly work with this semester are commonly human pathogens, we require that you read and agree to certain rules for working in the microbiology lab that are designed to keep you from infecting yourself, your classmates, and the community. We will also begin today to learn aseptic techniques that will reduce the chance of contaminating your cultures or the chance that your cultures will contaminate you.
Introduction To the Tools and Techniques of Microbiology
Whether you are trying to keep a desired organism from being overgrown by a contaminant, or you are attempting to prevent contaminating yourself, your lab bench, or your lab partner with your cultures; awareness of potential sources of contamination in a microbiology lab is critical. Your success in the lab depends on being open to learning and adopting the standard procedures used in microbiology. Today you will practice aseptic transfer technique and immediately assess your success.
Aseptic transfer
Please watch your instructors demonstrate aseptic transfer. You will be performing this transfer in the hoods in order to minimize contamination. After a demonstration by your instructor, you will practice the basics of aspetic transfer techniques and assess your success.
Broth to Broth or Broth to Plate Transfer
YouTube demo [1]
But keep in mind that in that demo they use a bunsen burner instead of an incinerator and also, we will be performing our inoculations in a laminar flow hood.
Protocol I: Broth to Broth
1. Label the destination container for the culture (uninoculated sterile broth in a tube or solid medium in a plate).
2. Holding your loop like a pencil, insert the loop into the incinerator. Keep the wire in the flame until it is red-hot. The wire will now be sterile. Allow the loop to cool for a few seconds in the air before touching it to your culture or medium.
3. Pick up the donor broth culture tube with your other hand, while still holding the sterile loop. With the hand holding the loop, use your little finger against your palm to remove the cover or plug from the culture tube as shown in Figure 1. Do not put the cover or plug down on your bench. Now, insert the loop into the broth without touching the sides of the tube, and then remove it, carrying a loopful of culture.

Figure 1: Transferring a culture. (a) Removal of a tube cap while manipulating a loop; (b) Obtaining inoculum from a broth tube while maintaining sterility of the cap (note cap in hand).
4. Pick up the labeled sterile destination tube or plate. Remove its cover.
5. Insert the loop containing the culture into the destination tube of sterile broth, swirl gently and remove. If the destination is a plate of solid medium, follow the directions for streaking for isolation or for a spread plate found below (part II).
6. Replace the cover and set the newly inoculated broth tube in the rack.
7. Re-sterilize the loop before putting it down by inserting the loop into the incinerator. Doing this slowly allows any liquid remaining on the loop to evaporate rather than boil and avoid splattering live bacterial cells all over the bench and you.
Protocol II: Broth to Plate
Nutrient agar plates can be streaked using a three-phase or a four phase pattern (Figure A-1). YouTube Demo [2]. The handling of the plate can be accomplished in a number of ways, all of which attempt to minimize possible contamination by either keeping the lid over the plate or by keeping the plate upside down (Figure A-2).
1. If using a loop, sterilize it first and allow the loop to cool for a few seconds. Not allowing any cap, lid, or plug to leave your hand, dip your loop in the donor broth culture or touch your loop to a colony of bacteria on a streak plate.
2. Using the loop, streak the first section of the plate using tight sweeping lines that stay within that section: (1/3 for a 3-phase pattern) or (1/4 for 4-phase) of the plate. It is fine to overlap your streaks in this section. (Fig A-3)
3. Sterilize the loop and allow it to cool in the air for 15 seconds. Touch the loop to an unused edge of the agar surface to cool it completely before continuing.
4. Pull the loop through the previous streak (section 1) one or two times to re-inoculate the loop with cells. Now streak section 2 of the plate, avoiding section 1 after the first 1-2 streaks and trying not to overlap the streaks (Figure A-4).
5. Sterilize the loop and allow it to cool in the air for 15 seconds. Touch the loop to an unused edge of the agar surface to cool it completely before continuing.
6. Pull the loop through one edge of the streak in section 2 of the plate to obtain inoculum. Now streak the 3rd section of the plate.
7. Sterilize the loop and repeat 6 and 7 until you complete inoculating all sections of your plate. Incubate and look for isolated colonies that have grown from one cell (Figure A-5).

Figure A-1: Two patterns for labeling the bottom of a plate for Isolation Streak technique. The inoculating loop is sterilized between each section and inoculum is taken from the preceding section to an uninoculated section of the plate.
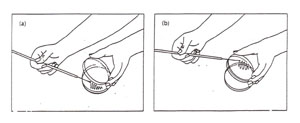
Figure A-2: Two options for aseptic transfer into a plate. (a) Streaking a plate while holding the lid ajar. Note that the lid shields the agar from airborne contamination: (b) streaking a plate while holding the bottom of the plate. Note that the agar surface faces downward, thereby minimizing contamination from the air.

Figure A-3: Pattern for isolation streaking section 1 of a plate.

Figure A-4: Illustration of isolation streak technique section 1 to section 2.

Figure A-5: An example of growth 24-72 hours after isolation streaking a plate to obtain isolated colonies.
Activity 1: Practice Exercise on Aseptic Transfer:
1. Obtain and label a tube of water using a piece of your team color tape (to be used as a label). On the tape write your initials and the date. Imagine that an organism is suspended in the stock tube (Tube A-found on your bench). Using Tube A and referring to the instructions for aseptic transfer (broth to broth), transfer a loopful of the contents of Tube A to a destination culture tube of water. (This first transfer is just for practice so you need not incubate this tube or save it for the next lab). Call your instructor over and ask them to assess your technique.
2. Label a destination nutrient agar plate with your initials, your lab section, the date, and the organism(s) or sample id. Always label on the AGAR side of plate. Why is it important to not label on the cover part of the plate? Cultures on solid media are usually incubated agar side up (covers down) to prevent condensation forming on the tops and then "raining" on your growing colonies, smearing them and making it more difficult to keep them separated. In addition, if 2 or more plate covers fall off accidentally, you will be unable to match the cover to the correct plate.
Tube B contains a mixed culture of bacteria in nutrient broth. Mix the bacteria by "flicking" or with a GENTLE vortex (do NOT invert it as these caps are purposely not air tight). Flame sterilize your loop and then insert the loop portion into the broth in Tube B. Remove the loop from the broth culture and lightly touch and drag the contaminated loop across section 1 of the destination plate. Follow the remaining instructions above for streaking to isolation.
Take the labeled plate you have inoculated to the designated rack for incubation..
Come back to the lab in a few days to check on your cultures. You hope to see well isolated colonies (two different looking types) in the last zones of your streak plate.
Bacterial Morphology
Background
The morphological characteristics of bacteria, including size, shape, and arrangement, can be seen by staining a bacterial smear so that individual bacterial cells are distinguished. For most species, morphologic characteristics are genetically determined and, thus, typical of the species. Generally, bacteria range in size from approximately 0.2 µm to 3.0 µm. The basic shapes are spherical (coccus), rod-shaped (bacillus), curved (vibrio), or helical (spirillum, spirochete). However, there are some species of bacteria that show considerable within species variation (termed pleomorphism). For example, both Mycoplasma (bacteria lacking the rigid cell wall of most bacteria) and Arthrobacter (a type of soil bacteria) show forms ranging from coccoid (round) to rodlike to filamentous. Some pathogenic species such as Mycobacterium tuberculosis and Corynebacterium diptheriae are also pleomorphic.
Bacteria are often individual but some species take on a group arrangement based on the way cell division and subsequent separation of the daughter cells occur. Most of the Gram-negative bacilli (rods) are found singly (Escherichia coli) or, sometimes, characteristically in pairs (Klebsiella pneumoniae). The cocci, Streptococcus pneumoniae and Neisseria are called diplococci because they tend to pair. Bacilli found in chains include some Bacillus species. The coccus genera that most often form chains are named Streptococcus for this defining arrangement. A few cocci make regular packets of four or eight (Micrococcus) and some are seen in irregular clumps that resemble bunches of grapes (Staphylococcus). Short rods that form parallel lines, called palisades, include the species that causes diptheria, Corynebacterium diptheriae. Chinese character formation describes a sharply angled bacterial arrangement. Because of their waxy cell walls, Mycobacterium species are difficult to emulsify and tend to stick together in clumps. The pathogen in this group, Mycobacterium tuberculosis, may form long cords of cells.
Keep in mind that individual cells may show deviations from these standard forms. For example, cocci of Neisseria show flattened sides, making them bean-shaped. The rods of Corynebacterium and Mycobacterium often appear club-shaped, with swollen ends or knobs. Both groups may show irregular staining. The diplococci of Streptococcus pneumoniae often appear slightly elongated and lancet-shaped (with one flattened end and one tapered end).
Making a Bacterial Smear
Activity 2: Prepare a Bacterial Smear Slide
Preparing a bacterial smear slide
0. Obtain cultures from your instructors -- cultures 1, 2 and 3.
1. Label a clean, glass slide with a graphite pencil on the far left of the slide with the number of the culture you will me smearing (The decolorizer in the Gram stain can remove your labels if you use pen or wax pencil.)
2. In the hoods, place one very small loopful of deionized water on the slide. (You can use the deionized water bottle on your bench; remove the cover and dip your loop in since sterility is not required for this step.
3. Sterilize the loop in the incinerator, allowing it to cool for a few seconds and then touch the cooled loop to a colony of your organism, picking up a TINY bit of growth from the bacterial colony. An invisible amount of growth obtained from just touching the cooled loop to the colony is fine. Too much picked up, such that the culture is a visible plug on the loop, is not fine.
4. Place the loop with the bacterial growth into the drop of water on the slide. Use a circular motion to make a smooth suspension of the bacteria in the water. Stop when there is a circle of emulsified bacteria about the size of a nickle on the slide.
5. Reflame the loop.
6. Repeat steps 1-4 with the other cultures.
7. Allow the slide to air dry completely! Be sure all the water on the slide has evaporated before proceeding to heat fixation!!! This drying step is crucially important. If you are impatient, you will "explode" the cells in the next step .
8. Heat fix (to kill and attach organisms to the slide) by quickly passing the slide (smear side up) through a flame 3 times. Your instructors will provide ethanol lamps or bunsen burners to use in the hoods for this purpose. Use a clothes pin or slide holder and avoid contact with hot glass.
An example of a multiple smear labeled slide:

The Gram Stain
Background on Using Stains in Bacteriology
The first of the dyes most useful to bacteriologists was a reddish violet dye, mauvein, synthesized in England by William. H. Perkin, and patented by him in 1856. This synthetic dye and others were immediately appreciated by histologists, but were not applied to bacterial cells until Carl Weigert (a cousin of Paul Ehrlich) used methyl violet to stain cocci in preparations of diseased tissue in 1875. Subsequently, the use of various synthetic dyes for bacteriological preparations developed rapidly when they were promoted through the publications of Robert Koch and Paul Ehrlich.
The synthetic dyes are classified as acid dyes, or basic dyes, depending on whether the molecule is a cation or an anion. The introduction of the terms acidic and basic was unfortunate because it would be more revealing to refer to them as cationic or anionic dyes. A look at the structural formula reveals the nature of the dye.

Each dye molecule has at least two functional chemical groupings. The auxochrome ionizes and gives the molecule the ability to react with the substrate, while the unsaturated chromophore absorbs specific wavelengths of light. The color of the solution obtained is that of the unabsorbed (transmitted) light. To be a dye, the molecule must have both auxochrome and chromophore groups. The auxochrome is usually an ionized carboxyl, hydroxyl, or pentavalent nitrogen group. The chromophore may have unsaturated nitrogen bonds such as azo (-N=N-) indamine (-N=), nitroso (-N=O) or nitro (O-N=O), groups; or unsaturated carbon to carbon, carbon to oxygen, or carbon to sulfur bonds, such as ethenyl (C=C), carbonyl (C=O), C=S, or the quinoid ring (= = =).
Resonance is also important to color. In crystal violet, an electron resonates between the three benzene rings. As the pH of the solution is lowered, the resonance becomes more and more restricted. When the resonance is restricted from three to only two benzene rings, the solution turns from violet to green, and then to red when resonance between the two rings ceases.
Cationic dyes will react with substrate groups that ionize to produce a negative charge, such as carboxyl, phenolic, or sulfhydryl groups. Anionic dyes will react with substrate groups which ionize to produce positive charges, such as the ammonium ion. Any substrate with such ionized groups should have an ability to combine with cationic or anionic dyes. Generally, the most important staining substrates in bacterial cells are proteins, especially the cytoplasmic proteins; however, other substances also have dye affinity. These include amino sugars, organic acids, nucleic acids, and certain polysaccharides.
Sudan III, or sudan black B, is a popular stain for fatty material. It does not have an auxochrome group, and is insoluble in water, but soluble in fatty material. When a solution of sudan black B in ethylene glycol is placed over bacterial cells, the fatty material will dissolve some of the dye and thus take on the color of the sudan black. The staining effect is purely a solubility phenomenon, and not a chemical reaction, or physical adsorption.
There are many stains that can reveal the morphology of the cell, and some simple stains, such as methylene blue, are quite good for viewing bacteria. The Gram stain is especially useful because it not only reveals bacterial morphology, but also is a differential stain. A differential stain differentiates organisms. (A differential stain shows a visible difference between different groups of organisms based on some characteristic they do not share, even though the procedure to stain the different looking organisms is the same). The Gram stain relies on cell wall differences between groups of bacteria.
The Gram staining procedure as it is done today, involves: a) primary staining of all cells with crystal violet, b) precipitating the primary stain dye within the cells with iodine (a mordant), c) removing the dye-iodine precipitate from some cells (the Gram-negative) with a decolorizer such as 95% ethanol, acetone, or n-propyl alcohol, and d) counter-staining of the decolorized cells with safranin. Organisms that retain the crystal violet primary dye are termed Gram-positive, while those which lose the primary stain and show the red safranin counter-stain are termed Gram-negative. This differentiation is not absolute, because it is based on the differences in the rate at which the primary dye is lost from the cells. If you over decolorize for too long or with too harsh a decolorizer, Gram-positive organisms will appear Gram-negative. Truly Gram-positive cells, such as Bacillus subtilis or Staphylococcus aureus, will not retain the primary dye if the iodine step is omitted. Criteria for a true Gram-positive state include the requirement of iodine following the crystal violet.
Activity 3: Preparing a Gram Stain
The Gram stain is a standard staining technique useful for the identification of culturable bacterial organisms and you will perform it now.
Use the slides prepared in Activity 2 and follow the Gram Stain Protocol found below. Your instructors will demonstrate how to perform a gram stain at the instructors bench.
Gram Stain Procedure:
To Gram stain the bacterial smear slide that you prepared in Activity 2, you must be careful to apply the staining reagents liberally so all the smears are evenly and completely covered and you must be sure to expose each smear to each reagent for the same amount of time.
1. Place your smear on the staining tray. It is important that the slide be level during staining so use paper towels under the tray to get it leveled. If you do, it is much easier to be sure that your smears will be covered evenly with each reagent.
2. Dispense just enough Crystal Violet solution (0.5% crystal violet, 12% ethanol, 0.1% phenol) to completely cover each smear and stain for 1 minute. (Crystal violet is the primary stain.)
3. Rinse the slide by lifting it at a 45 degree angle (using gloves or a clothes pin or slide holder) and use a squirt bottle to direct a very gentle stream of water slightly above the top smear. Rinse until the waste water coming off at the bottom is relatively clear; drain off excess water by touching the edge of the slide to a paper towel.
4. Dispense just enough Gram's Iodine (mordant)to completely cover each smear. Let stand for 1 minute. Rinse thoroughly with a gentle stream of water as in Step 1.
5. Lift the slide at a 45 degree angle and drip Decolorizing Reagent (80% isopropyl alcohol, 20% acetone) down the length of the slide making sure it comes in contact with all three smears. This step is tricky as it is easy to over- or under-decolorize. Do this for 10 seconds and IMMEDIATELY rinse, as in step 3, with a gentle stream of water.
6. Place the slide flat on the staining tray and dispense just enough Counterstain (0.6% safranin in 20% ethanol) to cover each smear. Let stand for 2 minutes; rinse with water as in step 3.
7. Blot dry using paper towels. Insert your slide between two paper towels and pat it dry.
8. Clean up your area; rinse your staining tray in the sink and leave it to drain upside down on paper towels.
9. Observe your stained microbes microscopically following the correct procedure for using the the oil immersion objective on your compound brightfield microscope. The directions for using the microscope are found below.
Use of the Compound Light Microscope
Activity 4: View your stained bacteria.
Today you will use only the 10x and 100x objectives. Remember also to read and follow the directions for care of this precision instrument (particularly on how to avoid getting immersion oil on any objective other than the 100x oil immersion lens). Be aware that there would be no field of microbiology if there weren't good, functioning microscopes to view this unseen world.The compound light microscope can magnify to about 1000 times the actual size of the specimen and can resolve details as fine as 0.2µm.
CARE OF THE MICROSCOPES
A compound microscope is available for each student's use. Remember at all times that your microscope is a precision optical instrument and must be handled carefully. When removing the microscope from its storage cabinet, do not jar or drop it, always carry it upright with one hand below the base and the other hand on the arm of the microscope. Place the microscope at least 6 inches from the edge of the bench. When returning the microscope to storage, check that:
1. the microscope light is turned off before the microscope is unplugged;
2. all lenses have been cleaned with lens paper, especially the oil immersion lens;
3. the lowest objective lens is near the stage and the stage itself is lowered; and
4. the microscope is covered (if there is a cover available).
PARTS OF THE MICROSCOPES
Figure 1 contains a diagram of a compound microscope and may help you locate some of the parts referred to in the following explanation. The compound microscope derives its name from the two sets of lenses it uses to magnify objects. These lenses are the objective lens, which can be found on the rotating nosepiece near the stage of the microscope, and the ocular lens, which is in the eyepiece. Your microscopes are equipped with several objective lenses, ranging from low to high magnification, including one oil immersion lens. The microscope magnifies by shining light from the light source through the iris diaphragm that limits the diameter of the light beam. The condenser lens focuses the light through the specimen that is on the stage. The stage is movable in order to view different parts of the specimen. The image we see is formed under the ocular lens by the objective lens and is a mirror image of the actual specimen.
REGULATION OF ILLUMINATION
1. The illumination intensity can be adjusted with a slider or with a knob.
2. Another way of adjusting illumination is by changing the position of the condenser lens. The condenser lens adjustment knob is located below the specimen stage and on the left side. It allows the user to move the condenser lens assembly up or down. As you move the condenser lens up, closer to the specimen, it concentrates (condenses) more light on your specimen. You will need to make this adjustment as you go up in magnification, so that you will have sufficient illumination.
3. The condenser aperture diaphragm is located below the specimen stage on the condenser lens assembly. It is an adjustable opening, which allows you to make fine adjustments in illumination. The lever, which adjusts the size of the aperture, faces the user. By sliding the lever to the left or right, you may adjust the illumination to the correct level for your specimen. Changing the size of this aperture also affects the amount of contrast in the image. Thus, adjusting the condenser aperture involves finding the brightness level, which gives you the best combination of illumination and contrast. This is the method used most often in adjusting illumination in the light microscope.
HOW TO LOCATE SPECIMENS USING A COMPOUND LIGHT MICROSCOPE
1. Place specimen slide on microscope stage and secure with clamping arm.
2. Use the 10x objective lens to find the specimen and focus it using first the coarse and then the fine adjustment knobs.
3. We will NOT use the 40x objective (high dry) because of the risk of getting immersion oil on that lens. Since bacteria are so small you should go directly to oil after focusing in low power by rotating the 10x objective away from the slide, putting a drop of oil on the center of the field of view, and then rotating the nosepiece so that the 100X objective lens comes in contact with the oil. Focus using the fine adjustment knob ONLY.
4. Never use the course adjustment when focusing a specimen with the oil objective because doing so could result in damage to the 100X objective lens or to the slide. All traces of oil must be removed from the lens before putting away the microscope. Only lens paper should be used to remove oil from the 100X objective.
CALCULATION OF TOTAL MAGNIFICATION
Total magnification of the specimen is determined by multiplying the magnifying power of the ocular and objective lenses. For example, a 10X ocular and a 100X objective together give a total magnification of 1,000X (Table 1). This means that the specimen appears 1000 times larger when viewed with a microscope than its actual size.
MEASUREMENT OF SIZE
Cell size can be measured using an ocular micrometer. A micrometer is sometimes found in one of the ocular lenses of each microscope. It looks like a small ruler with both large and small units. The large units are numbered 1, 2, 3, etc. The small units are subdivisions of the large units and are not numbered. There are 10 small units per large unit. The small units represent different lengths depending on the objective lens in use. You measure cellular structures in small units only, and then convert to metric units (µm = micrometers) using the conversion values below.
Therefore, if you are observing a cell with the 100X objective, and this cell spans 2.5 small units on the ocular micrometer scale, then the size of the cell is calculated by multiplying 2.5 small units x 1µm/small unit = 2.5µm.
Figure 1. A Cutaway Diagram showing the beam path of the Nikon Eclipse E200 Compound Light Microscope. [SOURCE: http://micro.magnet.fsu.edu/primer/anatomy/nikone200cutaway.html]
More Tools & Techniques of Microbiologists
Use and Calibration of Micropipets
How to Use a Micropipettor
The following website Using a Micropipette has more detailed information about micropipette use.
- Decide which of your micropipets is appropriate for the volume you want to measure and dispense.
- Adjust the volume dial to the appropriate volume, recognizing that your P1000 must have a zero added to the bottom of the volume display boxes and the P20 has a decimal between the bottom and second volume display boxes.
- Firmly seat a new micropipet tip of appropriate size on the micropipets.
- Depress with the thumb plunger to the first stop and hold the pipettor in this depressed position (DO NOT depress fully).
- Dip the micropipet into the solution far enough to account for the volume that will be withdrawn but not so far as to immerse the micropipet barrel.
- Gradually release the plunger, drawing fluid into the tip without forming bubbles.
- Carefully slide the micropipet tip along the side of the tube to remove any unwanted droplets of fluid sticking to the tip's surface.
- Expel the fluid into the desired container by touching the micropipet tip to the inside surface of the container and slowly depressing FULLY the plunger.
- Continue to hold the plunger in the fully depressed position as you remove the micropipet from the container.
- Eject the tip by pressing the eject button (if your micropipet has one) into an appropriate place (only tips that have been contaminated with microorganisms need to be ejected into an autoclave bag).
Don'ts in Using Micropipets
- DO NOT force or rotate the volume adjustment knob past the upper or lower ranges specified on the top of the micropipet.
- DO NOT use a micropipet without a tip since the precision piston that measures the volume can be ruined.
- DO NOT put the micropipet down on your bench with a filled tip since fluid can run back into the precision piston.
- DO NOT allow the micropipet plunger to snap back after fluid is either dispensed or drawn.
- DO NOT immerse the micropipet barrel into fluid.
- DO NOT flame the micropipet tip.
Activity 5: Protocol for Micropipet Calibration
1. To calibrate your P1000, P 200, and P 20 micropipets, label 6 microfuge tubes (1-6) and weigh them on a top loading balance. Don't forget to tare the balance. Record the weights in a table in your lab notebook, like the one below.
2. Using the information in the table below, pipet the specified volumes into the pre-weighed microfuge tubes prepared in step 1 and then reweigh the tubes. Record all weights.
3. Calculate the weight of the water in grams by substracting the dry weight from the weight of the tubes with water. Note that 1000 microliters of water should weigh exactly 1 gram at room temperature.
4. If the water in any tube weighs more or less than 1 gram, repeat that tube. Ask your instructor to check your pipetting technique if your calibration continues to be off after several repeated attempts.
| Tube # | Pre-weight | Tube Vol. in µl using P20 |
Vol. in µl using P200 |
Vol. in µl using P1000 |
Weight of water grams |
|---|---|---|---|---|---|
| 1 | _____ | 10 | 0 | 990 | ____ |
| 2 | _____ | 0 | 100 | 900 | ____ |
| 3 | _____ | 20 | 175 | 805 | _____ |
| 4 | _____ | 2 | 88 | 910 | ______ |
| 5 | _____ | 0 | 200 x 5 | 0 | _____ |
| 6 | _____ | 20 x 5 | 0 | 900 | _____ |
CLEAN UP
1. All culture plates that you are finished with should be discarded in the autoclave bag near the instructor table. Ask your instructor whether or not to save stock cultures and plates with organisms that are provided.
2. Culture plates, stocks, etc. that you are not finished with should be labeled on a piece of your your team color tape. Place the labeled cultures in your lab section's designated area in the incubator, the walk-in cold room, or at room temp. in a labeled rack. If you have a stack of plates, wrap a piece of your team color tape around the whole stack.
3. When done with liquid cultures, remove tape from all glass tubes. Then place the glass tubes with caps in racks by the sink near the instructor's table. Do not discard the contents of the tubes.
4. Glass slides or disposable glass tubes can be discarded in the glass disposal box.
5. Make sure all contaminated, plastic, disposable, serologic pipets and used contaminated micropipet tips are in non-hazardous sharps container.
6. If you used the microscope, clean the lenses of the microscope with lens paper, being very careful NOT to get oil residue on any of the objectives other than the oil immersion 110x objective. Move the lowest power objective into the locked viewing position, turn off the light source, wind the power cord, and cover the microscope with its dust cover before replacing the microscope in the cabinet.
7. If you used it, rinse your staining tray and leave it upside down on paper towels next to your sink.
9. Place all your equipment back where you found it at the beginning of the day.
10. Move your notebook and lab manual so that you can disinfect your bench thoroughly.
11. Wash your hands.




