Lidstrom:Electroporation
From OpenWetWare
Back to Protocols
- Electroporation is chosen when you need higher efficiency of transformation, or when you want to try transforming multiple plasmids at once.
- It is often difficult to get multiple plasmids in at once.
Preparing Electrocompetent Cells (in progress)
- Grow cells in LB (+ antibiotics, often)
- Wash 2 times with sterile 10% glycerol
- Resuspend in 10% glycerol
- If 50 mL of cells were used, ~ 2 mL is a reasonable volume.
- Aliquot into sterilized eppendorf tubes
- Flash freeze with liquid N2 and store at -80oC
Preparing Cuvettes
Chosing Cuvettes
- We bought these: these (7/24/2012) but are likely to switch to these.
- Originally, we wanted autoclavable cuvettes, however, it seems that ethanol sterilization is effective.
Sterilizing cuvettes for reuse
The cuvettes should be washed for reuse. If washed properly, they can be reused up to 10 times. For each cuvette, do the following while waiting for the cells to recover:
- Squirt 95% ethanol to fill cuvette more than halfway. Add cap and invert cuvette a few times to disinfect it. Pour out in waste receptacle.
- Wash cuvette three times with DI H2O using squeeze bottle and dump water into sink.
- Squirt 95% ethanol in the cuvette, add the cap, and let it sit for an hour or overnight.
- Pour out ethanol and place in UV hood to dry.
- Store in freezer for next use
It seems that the cuvettes rust when left to soak in solution, increasing their rate of arcing. Note: most cuvettes are made from plastic that will melt. Do not autoclave.
How much DNA do I use?
- Use 1 uL of Gibson assembly
- If you want to use more, do a PCR cleanup to remove salts, etc.
- plasmid miniprep: 100 pg (1/2 uL or less is usually sufficient)
- multiple plasmids at once: 5 ng each.
- We haven't had much success with this yet.
Transformig cells
- Add 1-10 μL of DNA source (see guide above) to the electrocompetent cells, swirl the tip gently in the culture to mix, and incubate on ice block for one minute.
- Working quickly, transfer the contents of the aliquot into the center of the gap of an ice-cold electrocuvette from the freezer, and then place the electrocuvette in the electroporator. Check that the electroporator is set to deliver 1.8 kV, and then press both of the orange buttons to deliver a pulse. The display will show "Ch 9" and eventually beep; release buttons when you hear this beep.
- Rob Egbert & the Klavins lab use 1.25 kV
- Again, working quickly, remove the electrocuvette from the electroporator and add 1/2 - 1 mL of room-temperature LB to it, pipetting up and down several times to extract the cells from the 1 mm gap.
- Transfer entire contents of electrocuvette back to the tube.
- Check and record the discharge time constant (units are in milliseconds).
- 4-5 seconds is good.
- Incubate tube transformed cells at 37°C for 30-60 minutes.
- Klavins lab does 30 - 45 min; our lab usually does 45 - 60 min.
- put plate in 37oC incubator with agar side up overnight
Misc
- settings for E. Coli recommended by BioRad customer support:
- brown-caped 1 mm gap tubes:
- 1.8 kV, 200 Ohms, 25 uF.
- green-capped 2 mm cuvette: (in case you don't have brown cuvettes ready)
- 2.5 to 3.0 KV, 200 Ohms, 25 uF
- brown-caped 1 mm gap tubes:
- Alternately, add ~950 uL ice cold SOC media to cuvette after shocking
- Optional: after shocking, chill sample on ice for 2 mins to permit the cells to recover.
- Optional: plate transformation onto prewarmed LB-agar plate supplemented with appropriate antibiotic. I generally plate 200μL but appropriate plating volume depends on efficiency of the transformation.
- Leave remaining SOC-cell mixture on the benchtop overnight.
- If you don't have any transformants, plate the rest of the transformation in the morning.
- Note: you will usually get plenty of colonies by using TB (not SOC media, not chilling cuvettes, not icing sample after applying voltage, not pre-warming plates.
- When choosing the # of vials and cuvettes, you can include 2 vials for two negative controls (one with no DNA added, and another with only cut vector added).
- Cuvette types: (see pic on right)
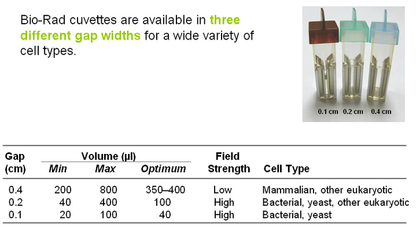
- BioRad Movie about electroporation. Though it is made for a different machine than we have, the principles are applicable.