CH391L/S13/Synthetic Meats and Organs: Difference between revisions
No edit summary |
|||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 7: | Line 7: | ||
[[Image:CH391L_S13_In_Vitro_Process.jpg|thumb|right|This picture depicts a snapshot of in vitro meat production.]] | [[Image:CH391L_S13_In_Vitro_Process.jpg|thumb|right|This picture depicts a snapshot of in vitro meat production.]] | ||
Since the advent of tissue engineering, the desire to create lab-grown replacement organs accrued in order to fulfill the needs of transplant patients. When organs | Since the advent of tissue engineering, the desire to create lab-grown replacement organs has accrued significantly in order to fulfill the needs of transplant patients. When organs deteriorate or fail due to issues like disease or adverse drug treatment effects the need for transplantable organs arise, but the demand is not always satisfied due to a lack of donors. In fact, over 100,000 patients are on the recipient waiting list and over 6,000 people die from the lack of a donor organ every year. | ||
Although there are enough donor organs to be transplanted, compatibility is a major issue. In vitro organs would be proper solution because these organs can be grown tailored specifically to each patient. In vitro organs are organs grown outside the body. In vitro organs are also known as synthetic organs. The main objective of research in synthetic organs is to maintain and direct the overall development of the organ's architecture. | |||
Incidentally, the offshoot topic of in vitro meats received popular attention concomitantly with in vitro organs. In vitro meats currently has not prevalence in today's economy because simply there is no demand for synthetic meats. Most people who indulge in nonvegetarian food are complacent of the environmental impact and even the preparation of their meat. While, vegetarians and vegans do not support such an option due to its unavailability. Without a demand, in vitro meats research cannot find the capital to industrialize and mass produce synthetic meats. As of now (2013), an in vitro meat hamburger is estimated to cost $330,000. The only hope for this possible industry is to take advantage of the skyrocketing prices of meats, namely beef. Although the potentials of in vitro meats can solve many societal issues, in vitro meats research needs to address certain pitfalls. In vitro meats are cultured muscle tissue with the purpose of replacing dietary meat. In Vitro meats are known as synthetic meats, test tube meat, tube steak, cultured meat, hydroponic meat, vat-grown meat, victimless meat and shmeat.<cite>Benjaminson2002</cite> | Incidentally, the offshoot topic of in vitro meats received popular attention concomitantly with in vitro organs. In vitro meats currently has not prevalence in today's economy because simply there is no demand for synthetic meats. Most people who indulge in nonvegetarian food are complacent of the environmental impact and even the preparation of their meat. While, vegetarians and vegans do not support such an option due to its unavailability. Without a demand, in vitro meats research cannot find the capital to industrialize and mass produce synthetic meats. As of now (2013), an in vitro meat hamburger is estimated to cost $330,000. The only hope for this possible industry is to take advantage of the skyrocketing prices of meats, namely beef. Although the potentials of in vitro meats can solve many societal issues, in vitro meats research needs to address certain pitfalls. In vitro meats are cultured muscle tissue with the purpose of replacing dietary meat. In Vitro meats are known as synthetic meats, test tube meat, tube steak, cultured meat, hydroponic meat, vat-grown meat, victimless meat and shmeat.<cite>Benjaminson2002</cite> | ||
| Line 19: | Line 20: | ||
===The Controversy=== | ===The Controversy=== | ||
In an environmental standpoint, in vitro meats would cut down the carbon emissions caused by the slaughtering cows and producing cow feed. The raising, slaughtering and disposing of cows lead to the release of carbon via methane or fossil fuels. Also, the requirement of feed forces toll on the environment which leads to the inefficient use of land. For every cow’s death, an estimated equivalent of 36.4 of carbon dioxide is produced. World meat production | In an environmental standpoint, in vitro meats would cut down the carbon emissions caused by the slaughtering cows and producing cow feed. The raising, slaughtering and disposing of cows lead to the release of carbon via methane or fossil fuels. Also, the requirement of feed forces toll on the environment which leads to the inefficient use of land. For every cow’s death, an estimated equivalent of 36.4 of carbon dioxide is produced. World meat production contributes about 20% of total carbon emissions. With in vitro meats, the need for slaughter houses and grazing lands would be diminished, focusing such lands to be placed under other roles. Even some look this supposed alternative solution skeptically, proclaiming that the mass production of in vitro meat will be counterintuitive in terms of carbon emissions.<cite>Fiala2008</cite> | ||
==Meat and Organ Production== | ==Meat and Organ Production== | ||
| Line 38: | Line 39: | ||
[[Image:CH391L_S13_trachea.jpg|thumb|left|The first surgically transplanted in vitro organ by Dr. Paolo Macchiarini. The trachea is composed of stem cells and plastics.]] | [[Image:CH391L_S13_trachea.jpg|thumb|left|The first surgically transplanted in vitro organ by Dr. Paolo Macchiarini. The trachea is composed of stem cells and plastics.]] | ||
One particular method to grown in vitro organs is known as '''decellularization'''. Decellularization is a tissue technique where all cells are removed from a damaged organ so that the extracellular proteins are left. These proteins are known as the extracellular matrix which retains the biological information of the decellularized organ. This information includes size, shape and even function. If stem cells are placed in the extracellular matrix, then the stem cells will adopt the true shape of the original, functioning organ based on cues from the extracellular proteins. Current research, spearheaded by Dr. Doris Taylor, is attempting to optimize the efficiency of synthetic hearts; currently synthetic hearts grown via decellularization are only 20% efficient. | One particular method to grown in vitro organs is known as '''decellularization'''. Decellularization is a tissue technique where all cells are removed from a damaged organ so that the extracellular proteins are left. These proteins are known as the extracellular matrix which retains the biological information of the decellularized organ. This information includes size, shape and even function. If stem cells are placed in the extracellular matrix, then the stem cells will adopt the true shape of the original, functioning organ based on cues from the extracellular proteins. Current research, spearheaded by Dr. Doris Taylor, is attempting to optimize the efficiency of synthetic hearts; currently synthetic hearts grown via decellularization are only 20% efficient. <cite>Carrier1999</cite> | ||
# Special detergent to remove (decellularize) cells | # Special detergent to remove (decellularize) cells | ||
Revision as of 20:17, 25 March 2013
Introduction to Synthetic Meats and Organs
"Fifty years hence, we shall escape the absurdity of growing a whole chicken in order to eat the breast or wing, by growing these parts separately under a suitable medium." - Winston Churchill

Since the advent of tissue engineering, the desire to create lab-grown replacement organs has accrued significantly in order to fulfill the needs of transplant patients. When organs deteriorate or fail due to issues like disease or adverse drug treatment effects the need for transplantable organs arise, but the demand is not always satisfied due to a lack of donors. In fact, over 100,000 patients are on the recipient waiting list and over 6,000 people die from the lack of a donor organ every year. Although there are enough donor organs to be transplanted, compatibility is a major issue. In vitro organs would be proper solution because these organs can be grown tailored specifically to each patient. In vitro organs are organs grown outside the body. In vitro organs are also known as synthetic organs. The main objective of research in synthetic organs is to maintain and direct the overall development of the organ's architecture.
Incidentally, the offshoot topic of in vitro meats received popular attention concomitantly with in vitro organs. In vitro meats currently has not prevalence in today's economy because simply there is no demand for synthetic meats. Most people who indulge in nonvegetarian food are complacent of the environmental impact and even the preparation of their meat. While, vegetarians and vegans do not support such an option due to its unavailability. Without a demand, in vitro meats research cannot find the capital to industrialize and mass produce synthetic meats. As of now (2013), an in vitro meat hamburger is estimated to cost $330,000. The only hope for this possible industry is to take advantage of the skyrocketing prices of meats, namely beef. Although the potentials of in vitro meats can solve many societal issues, in vitro meats research needs to address certain pitfalls. In vitro meats are cultured muscle tissue with the purpose of replacing dietary meat. In Vitro meats are known as synthetic meats, test tube meat, tube steak, cultured meat, hydroponic meat, vat-grown meat, victimless meat and shmeat.[1]
History
Arguably, research conducted by the National Aeronautical Space Administration (NASA) into in vitro meats paved a way to encourage the scientific community to further the progress. In 1995, the Food and Drug Admisnistration (FDA) approved the techniques to produce such in vitro meats. In 2001, NASA cultured in vitro meats from turkey cells. But, the first edible form of in vitro meat was grown from goldfish by the Tuoro Applied Bioscience Research Consortium in 2000. Apparently, the in vitro meat was grown to resemble fish fillets. In 2001, Willem van Eelen M.D., Wiete Westerhof Ph.D., and Willem van Kooten filed for a world wide patent on a process to produce in vitro meat which required a matrix collagen to be seeded with muscled cells. In 2004, Jon F. Vein also held a patent (U.S. Patent 6,835,390) for the production of in vitro meat for human consumption from muscle and fat cells. Research in synthetic meats was encouraged by People of Ethical Treatment of Animals (PETA) who offered a million dollar prize to the first company to offer in vitro meats to consumers. Without a demand for in vitro meats, in vitro meats would not make little headway; therefore, this prize that was announced in 2008 greatly fuels the race to industrialize this field. The deadline for the prize has been extended to some date in 2013.
The history of in vitro organs is not as colorful since much progress has yet to be made. In vitro organs research evolves as new techniques in tissue engineering have been uncovered. On June 9, 2011, the first synthetic organ was transplanted into a human subject with trachea cancer. A trachea composed from stem cells was placed into a 36 year old man at Karolinska University Hospital in Stockholm. This is a huge step forward in the in vitro organs research.
The Controversy
In an environmental standpoint, in vitro meats would cut down the carbon emissions caused by the slaughtering cows and producing cow feed. The raising, slaughtering and disposing of cows lead to the release of carbon via methane or fossil fuels. Also, the requirement of feed forces toll on the environment which leads to the inefficient use of land. For every cow’s death, an estimated equivalent of 36.4 of carbon dioxide is produced. World meat production contributes about 20% of total carbon emissions. With in vitro meats, the need for slaughter houses and grazing lands would be diminished, focusing such lands to be placed under other roles. Even some look this supposed alternative solution skeptically, proclaiming that the mass production of in vitro meat will be counterintuitive in terms of carbon emissions.[2]
Meat and Organ Production
New Aged Butcher Shop
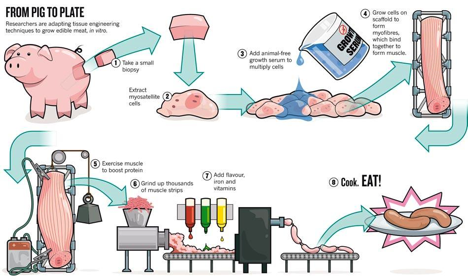
In brief, there are currently two proposed approaches in the production in vitro meat: embryonic stem cells and satellite cells (progenitor muscle cells). Initially, muscles cells start with mononucleated myoblast of limited cellular division properties. As these myoblasts fuse into multinucleated myotubes, muscle fibers are created without proliferative properties. Both embryonic stem cells and satellite cells give rise to myofibers, but their approached are fundamentally different. Embryonic stem cells are favorable for their unlimited proliferative ability but may inaccurately restart myogenesis. Contrastingly, satellite cells are favorable ability to accurately restart myogenesis but have limited proliferative properties. In a way, this is a fundamental debate of quality versus quantity. Nevertheless, either embryonic stem cells or satellite cells are cultured in a bioreactor in order for the cells to fuse into muscle fibers which requires a perfusion system. The perfusion system supplied by the bioreactor can deliver both nutrients and oxygen while expelling wastes[3]. The scaffold system directs the growth of the myofibers. The scaffold based method produces a thin myocyte layers at a time, hence the name shmeat- sheets and meat. There isn’t a need for a specific scaffold system, but current research favors the collagen matrix to aid in shaping the meat. The scaffold only needs to be as tough as the meat produced. Lastly, the muscle needs to be excercised for optimal growth.
- Cells: Embryonic Stem Cells and Satellite cells
- Scaffold: Collagen meshwork and collagen beads
- Culture condition: serum or nonserum
- Bioreactor: standard
- Oxygen perfusions: depends on culture condition[4]
Organ Hatchery

One particular method to grown in vitro organs is known as decellularization. Decellularization is a tissue technique where all cells are removed from a damaged organ so that the extracellular proteins are left. These proteins are known as the extracellular matrix which retains the biological information of the decellularized organ. This information includes size, shape and even function. If stem cells are placed in the extracellular matrix, then the stem cells will adopt the true shape of the original, functioning organ based on cues from the extracellular proteins. Current research, spearheaded by Dr. Doris Taylor, is attempting to optimize the efficiency of synthetic hearts; currently synthetic hearts grown via decellularization are only 20% efficient. [5]
- Special detergent to remove (decellularize) cells
- Endonuclease to degrade floating nucleic acid
- the extracellular matrix is protected and preserved until stem cell treatment[6]
IGEM Take Home Message
In 2010 IGEM competition, MIT underwent a project that sought out smart tissue that would proliferate in response to mechanical stress. Essentially, MIT presents a new approach to in vitro organ growth without the need of a scaffold. Specifically, in vitro bone tissue was created to sense mechanical stress and differentiate accordingly. Moreover, where more mechanical stress is found, the bone density in that region would be higher to compensate for the high stress. This smart in vitro bone tissue contains mechanoreceptor promoters that are able to sense the stress. This promoter regions are RE/CRE2 and NR1/2 that confer sensitivitiy to shear stress in shake tests.[7]
References
- Benjaminson MA, Gilchriest JA, and Lorenz M. In vitro edible muscle protein production system (MPPS): stage 1, fish. Acta Astronaut. 2002 Dec;51(12):879-89. DOI:10.1016/s0094-5765(02)00033-4 |
This paper could be a possible paper to share for discussion for in vitro meat research, but according to sources the methodology describes is not useful for mass production.
- Carrier RL, Papadaki M, Rupnick M, Schoen FJ, Bursac N, Langer R, Freed LE, and Vunjak-Novakovic G. Cardiac tissue engineering: cell seeding, cultivation parameters, and tissue construct characterization. Biotechnol Bioeng. 1999 Sep 5;64(5):580-9. DOI:10.1002/(sici)1097-0290(19990905)64:5<580::aid-bit8>3.0.co;2-x |
- Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, and Taylor DA. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008 Feb;14(2):213-21. DOI:10.1038/nm1684 |
This is another paper for discussion of decellularization and in vitro organs