CH391L/S13/GeneticMarkers: Difference between revisions
No edit summary |
No edit summary |
||
| Line 11: | Line 11: | ||
Perhaps the most commonly used selectable marker in bacteria is the ''ampR'' gene, which provides resistance to certain beta-lactam antibiotics such as ampicilllin (amp) and its more stable relative carbenicillin (carb). Beta-lactam antibiotics are penicillin derivatives that inhibit synthesis of bacterial peptidoglycan cell walls, arresting cell division and ultimately leading to cell death. Although beta-lactams are primarily functional against gram-positive bacteria due to their larger cell walls, some examples, such as amp and carb, are capable of killing gram-negative E. coli. All antibiotics in the family share a central four atom ring structure known as the beta-lactam ring, which serves as a cleavage target for enzymes known as beta-lactamases. Due to the shared structural features of becta-lactam antibiotics, these enzymes often have promiscuous activities that target multiple drugs. Upon cleavage of the lactam ring, antibiotics such as ampicillin are no longer toxic to bacterial cells, allowing cell growth and division to resume. | Perhaps the most commonly used selectable marker in bacteria is the ''ampR'' gene, which provides resistance to certain beta-lactam antibiotics such as ampicilllin (amp) and its more stable relative carbenicillin (carb). Beta-lactam antibiotics are penicillin derivatives that inhibit synthesis of bacterial peptidoglycan cell walls, arresting cell division and ultimately leading to cell death. Although beta-lactams are primarily functional against gram-positive bacteria due to their larger cell walls, some examples, such as amp and carb, are capable of killing gram-negative E. coli. All antibiotics in the family share a central four atom ring structure known as the beta-lactam ring, which serves as a cleavage target for enzymes known as beta-lactamases. Due to the shared structural features of becta-lactam antibiotics, these enzymes often have promiscuous activities that target multiple drugs. Upon cleavage of the lactam ring, antibiotics such as ampicillin are no longer toxic to bacterial cells, allowing cell growth and division to resume. | ||
[[Image:amp.png|center| Ampicillin]] | [[Image:amp.png|thumb|center|500px| The structure of ampicillin, including the central beta-lactam ring cleaved by the ''ampR'' gene product [http://en.wikipedia.org/wiki/Ampicillin Ampicillin on Wikipedia]]] | ||
The common ''ampR'' gene, as used in the ''E. coli'' pBR322 plasmid, was naturally derived from ''Salmonella'' bacteria through transposition. <cite>Sutcliffe78</cite> | The common ''ampR'' gene, as used in the ''E. coli'' pBR322 plasmid, was naturally derived from ''Salmonella'' bacteria through transposition. <cite>Sutcliffe78</cite> | ||
Revision as of 06:40, 4 March 2013
Introduction
The ability to introduce exogenous DNA into an organism to alter its genetic program is one of the most crucial tools in modern biology. Early work showed that certain bacteria could acquire the traits of a related strain through the addition of heat-killed cells. Although it was not well understood at the time, the transfer of gene-encoding DNA from one strain to another facilitated this. This concept was turned into a useful tool upon the advent of bacterial plasmid transformations in the early 1970's, which allowed genes of interest to be easily inserted into E. coli. Over the years, methods have been developed to introduce exogenous genes into a wide range of useful organisms, including bacteria, yeasts, plants, and animal tissues. These methods vary enormously in efficiency however, necessitating a way to identify and isolate cells which contain the DNA of interest. This can be accomplished either by screening for successfully modified cells, or through selection.
Screening vs. Selection
In the case of bacterial transformations, it's possible to plate cells post-transformation on non-selective media and screen each individual colony to identify those that have been successfully modified. Because each colony arises from a single cell present during transformation, the colony is made up of a set of identical clones. Screening can involve testing for the desired DNA itself or for the product of an inserted gene. This process is extremely difficult however, as the vast majority of cells will not be successfully transformed, so many colonies need to be tested to identify even a single transformant. A much more efficient strategy is to use a selectable genetic marker that allows only those cells which have been transformed to survive under certain growth conditions. These marker genes may be combined with the DNA of interest on a single plasmid, thus ensuring that any cells that survive selection contain the gene(s) of interest. The most commonly used selectable markers in bacteria are genes that provide resistance to a specific antibiotic upon transformation, allowing for positive selection of cells containing the marker.
Antibiotic Resistance Markers
ampR
Perhaps the most commonly used selectable marker in bacteria is the ampR gene, which provides resistance to certain beta-lactam antibiotics such as ampicilllin (amp) and its more stable relative carbenicillin (carb). Beta-lactam antibiotics are penicillin derivatives that inhibit synthesis of bacterial peptidoglycan cell walls, arresting cell division and ultimately leading to cell death. Although beta-lactams are primarily functional against gram-positive bacteria due to their larger cell walls, some examples, such as amp and carb, are capable of killing gram-negative E. coli. All antibiotics in the family share a central four atom ring structure known as the beta-lactam ring, which serves as a cleavage target for enzymes known as beta-lactamases. Due to the shared structural features of becta-lactam antibiotics, these enzymes often have promiscuous activities that target multiple drugs. Upon cleavage of the lactam ring, antibiotics such as ampicillin are no longer toxic to bacterial cells, allowing cell growth and division to resume.
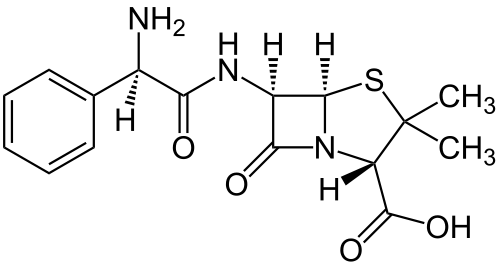
The common ampR gene, as used in the E. coli pBR322 plasmid, was naturally derived from Salmonella bacteria through transposition. [1]
tetA
Cap
Non-Antibiotic Markers=
Novel Marker Strategies
TetA Dual Genetic Selection
References
- Sutcliffe JG. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3737-41. DOI:10.1073/pnas.75.8.3737 |
Background on the ampR gene from pBR322
- Muranaka N, Sharma V, Nomura Y, and Yokobayashi Y. An efficient platform for genetic selection and screening of gene switches in Escherichia coli. Nucleic Acids Res. 2009 Apr;37(5):e39. DOI:10.1093/nar/gkp039 |
Riboswitch selection/screening using a tetA-GFP fusion marker