CH391L/S13/DnaAssembly: Difference between revisions
Gabriel Wu (talk | contribs) |
Gabriel Wu (talk | contribs) |
||
| Line 63: | Line 63: | ||
EcoRI digestion produces "sticky" ends, | EcoRI digestion produces "sticky" ends, | ||
[http://en.wikipedia.org/wiki/File:EcoRI_restriction_enzyme_recognition_site.svg] <br> | [http://en.wikipedia.org http://en.wikipedia.org/wiki/File:EcoRI_restriction_enzyme_recognition_site.svg] <br> | ||
[[Image:EcoRI restriction enzyme recognition site.svg|90px]] | [[Image:EcoRI restriction enzyme recognition site.svg|90px]] | ||
Revision as of 02:50, 28 January 2013
DNA synthesis and molecular cloning are tools used by synthetic biologists to create the biological "parts" needed to design and engineer biological devices and systems.
Introduction
As described before, synthetic biology captures a diverse, multi-disciplinary field. No matter which definition(s) becomes accepted, the ability to make and manipulate DNA is a vital component to practicing synthetic biology.
A large number of parts have been made by the synthetic biology community. Many can be found as part of the Registry of Standard Biological Parts. These modular genetic components are designed to be easy to acquire and assemble to facilitate the building of more complex biological devices. To learn more about the Registry and the biological parts known as BioBricks™, see the entry for the iGEM Registry.
The Registry of Standard Biological Parts is an attempt to create an annotated and characterized repository of biological parts. It is motivated in part because synthetic biologists rely on the ability to make testable biological units. While the parts registry is a useful resource, it is not comprehensive. The ability to manipulate and create genetic material is a necessary skill for being a successful synthetic biologists. This page details how to create DNA from small (<60 nts) oligonucleotides to larger genes (~400 nts) to genome sized (~500 ,000 nts) biological units. Many of the methods found here are the basis for the construction of the registry itself.
DNA Synthesis
Oligonucleotide Synthesis
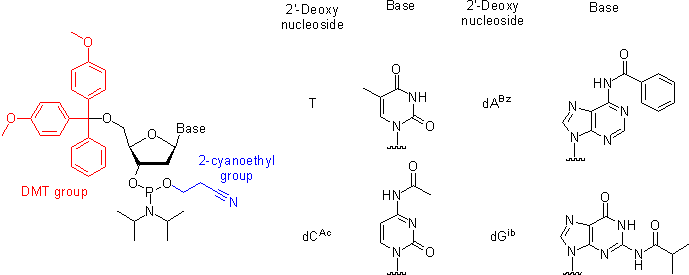
Oligonucleotides are chemically synthesized from DNA phosphoramidite monomers. Briefly, activated phosphoramidite monomers are added in the 3' to 5' direction using a cyclical activation and blocking chemistry to obtain a DNA polymer linked by phosphodiester bonds.
Chemical synthesis is currently limited to oligonucleotides of about 200 nt in length.
Gene Synthesis
Gene synthesis, or artificial gene synthesis, refers to the process of creating a nucleic acid template for a gene in vitro, without the requirement of a preexisting DNA template. Soon after the elucidation of the genetic code and the description of the central dogma of molecular biology, there arose a need to synthesize genes de novo in order to study their biological function both in the test tube and in model organisms. Chemical synthesis of DNA has grown from an expensive and time-consuming process into a viable commercial industry capable of high-throughput manufacture of almost any scale of custom DNA molecules in almost any context. This allows species-specific gene optimization, creation of genes from rare or dangerous sources, and combinatorial assembly of any DNA sequence that can be chemically synthesized, even including non-traditional bases. The most advanced applications of gene synthesis have been applied to the recent creation of completely synthetic minimal genomes in prokaryotes.
Despite nearly four decades of progress in gene synthesis technologies, most DNA sequences used in modern molecular biology are assembled in part or in whole from naturally occurring templates. However this limits the scope and applications to previously existing genes and the results of large-scale genomic surveys of novel genes from nature. Modern gene synthesis relies heavily on advancements in chemical DNA oligonucleotide synthesis, with the primary challenges being scale, cost, fidelity and the eventual assembly of complete gene products.
An extensive, but not comprehensive, directory of commercial gene synthesis providers can be found at Genespace.
Economics


History of Gene Synthesis
Gene synthesis predates the invention of restriction enzymes and molecular cloning techniques by several years. The first gene to be completely synthesized in vitro was a 77-nt alanine transfer RNA by the laboratory of Har Gobind Khorana in 1972 [1]. This was the result of nearly five years of work and resulted in a DNA template without promoter or transcriptional control sequences. The first peptide- and protein-producing synthetic genes were created in 1977 and 1979, respectively [2, 3]. Steady advancement has led to recent synthesis of complete gene clusters tens of thousands of nucleotides in length, and ultimately a bacterial genome approximately 1.1 million bases in length [4].

Molecular Cloning
It is impractical for most synthetic biologists to synthesize more than several kilobases of completely synthetic DNA. It is often desirable to build bigger pieces at lower costs and faster speeds than de novo synthesis is currently able to accomplish.
Over the years, many different strategies have been developed to assemble DNA in flexible ways that suit different purposes. These strategies typically employ purification of enzymes that are known to modify DNA in specific ways and these methods of action can be exploited for designing and building specific sequences of DNA. For example, restriction enzymes are used to cut DNA in a specific manner upon recognition of a specific nucleotide sequence. Polymerases and endonucleases add or remove nucleotides to make double stranded DNA from a single stranded template or to create single stranded DNA from double stranded DNA. Many of the modern techniques take advantage of recombination machinery that break DNA from one location to reattach it to another location. In other cases, the endogenous enzymes in a host are utilized to manipulate DNA without the need for prior purification. For example, in some methods endogenous DNA ligase is used to repair single stranded breaks (known as "nicks") to complete the formation of fully circular DNA.
Ultimately, these methods generally require transformation into a host where endogenous enzymes are used to complete the genetic manipulation and replicate (clone) the genetic material. This allows for the expression of the desired proteins to test the ability of the engineered system or for the purification of the genetic material itself such that it can be used for further manipulation, study, or storage.
While there are many specific protocols for the numerous methods of cloning, most share reasonable overlap in their underlying mechanisms of action. Broadly speaking, methods may rely primarily on restriction enzymes, polymerase chain reaction (PCR), or on homologous recombination.
Restriction Enzyme
Restriction enzymes recognize a specific nucleotide sequence and then cut the DNA in such a way that results in a double stranded break. If the enzyme cuts within the recognition site, it is classified as a Type I restriction enzyme. If the enzyme cuts outside of the restriction site, the enzyme is classified as a Type II restriction enzyme. Restriction enzymes can also be classified by if the DNA break results in a straight cut resulting in a blunt end or with a jagged cut resulting in a sticky end.
For example: EcoRI digestion produces "sticky" ends,
http://en.wikipedia.org/wiki/File:EcoRI_restriction_enzyme_recognition_site.svg
File:EcoRI restriction enzyme recognition site.svg
whereas SmaI restriction enzyme cleavage produces "blunt" ends:
File:SmaI restriction enzyme recognition site.svg
The Registry of Biological Parts has used the notion of
- BioBricks
- BglBricks
list of BioBrick Foundation Standards
- CpoI directional cloning
- golden gate
- MoClo [5]
- GoldenBraid
Polymerase Chain Reaction
Ligation Independent Cloning (LIC) [6]
TOPO TA Cloning (Invitrogen) [7][8]
Splice by Overlap Extension (SOE) PCR [9]
Polymerase Cycling Assembly (PCA) [10]
Recombination/Homology
- In-Fusion (Clontech) poxvirus DNA polymerase with 3′–5′ exonuclease activity [11][12]
- In-Fusion BioBrick Assembly [13]
- cold fusion (SBI)
- Cre/Lox P1 phage (Clontech)
- att lambda (gateway)
- CloneEZ kit (Genescript) [2], recombination around a linearized vector
- GENEART Seamless Cloning (Life Technologies previously Invitrogen previously DoGene)
- SLIC sequence and ligation independent cloning T4 DNA polymerase (exonuclease)
- Gibson T5 exonuclease, Phusion polymerase, Taq ligase
- CPEC circular polymerase extension cloning
- SLiCE (Seamless Ligation Cloning Extract) in vitro homologous recombination
In Vivo
-MAGIC (bacterial mating) [14]
-Recombineering lambda red
More cloning strategies found here
Links of Interest
- Gibthon - Gibson assembly design program
- j5: A tool for designing DNA assembly with recombination from Nathan Hillson The manual has an excellent overview of recombination-based cloning strategies like SLIC and Gibson. [15]
References
- Khorana HG, Agarwal KL, Büchi H, Caruthers MH, Gupta NK, Kleppe K, Kumar A, Otsuka E, RajBhandary UL, Van de Sande JH, Sgaramella V, Terao T, Weber H, and Yamada T. Studies on polynucleotides. 103. Total synthesis of the structural gene for an alanine transfer ribonucleic acid from yeast. J Mol Biol. 1972 Dec 28;72(2):209-17. DOI:10.1016/0022-2836(72)90146-5 |
- Itakura K, Hirose T, Crea R, Riggs AD, Heyneker HL, Bolivar F, and Boyer HW. Expression in Escherichia coli of a chemically synthesized gene for the hormone somatostatin. Science. 1977 Dec 9;198(4321):1056-63. DOI:10.1126/science.412251 |
- Goeddel DV, Kleid DG, Bolivar F, Heyneker HL, Yansura DG, Crea R, Hirose T, Kraszewski A, Itakura K, and Riggs AD. Expression in Escherichia coli of chemically synthesized genes for human insulin. Proc Natl Acad Sci U S A. 1979 Jan;76(1):106-10. DOI:10.1073/pnas.76.1.106 |
- Gibson DG, Glass JI, Lartigue C, Noskov VN, Chuang RY, Algire MA, Benders GA, Montague MG, Ma L, Moodie MM, Merryman C, Vashee S, Krishnakumar R, Assad-Garcia N, Andrews-Pfannkoch C, Denisova EA, Young L, Qi ZQ, Segall-Shapiro TH, Calvey CH, Parmar PP, Hutchison CA 3rd, Smith HO, and Venter JC. Creation of a bacterial cell controlled by a chemically synthesized genome. Science. 2010 Jul 2;329(5987):52-6. DOI:10.1126/science.1190719 |
genome replacement
- Weber E, Engler C, Gruetzner R, Werner S, and Marillonnet S. A modular cloning system for standardized assembly of multigene constructs. PLoS One. 2011 Feb 18;6(2):e16765. DOI:10.1371/journal.pone.0016765 |
MoClo
- Aslanidis C and de Jong PJ. Ligation-independent cloning of PCR products (LIC-PCR). Nucleic Acids Res. 1990 Oct 25;18(20):6069-74. DOI:10.1093/nar/18.20.6069 |
LIC
- Holton TA and Graham MW. A simple and efficient method for direct cloning of PCR products using ddT-tailed vectors. Nucleic Acids Res. 1991 Mar 11;19(5):1156. DOI:10.1093/nar/19.5.1156 |
TA cloning
- Shuman S. Recombination mediated by vaccinia virus DNA topoisomerase I in Escherichia coli is sequence specific. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10104-8. DOI:10.1073/pnas.88.22.10104 |
TOPO
- Higuchi R, Krummel B, and Saiki RK. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988 Aug 11;16(15):7351-67. DOI:10.1093/nar/16.15.7351 |
SOE-PCR
- Stemmer WP, Crameri A, Ha KD, Brennan TM, and Heyneker HL. Single-step assembly of a gene and entire plasmid from large numbers of oligodeoxyribonucleotides. Gene. 1995 Oct 16;164(1):49-53. DOI:10.1016/0378-1119(95)00511-4 |
PCA
- Zhu B, Cai G, Hall EO, and Freeman GJ. In-fusion assembly: seamless engineering of multidomain fusion proteins, modular vectors, and mutations. Biotechniques. 2007 Sep;43(3):354-9. DOI:10.2144/000112536 |
in-fusion
- Benoit RM, Wilhelm RN, Scherer-Becker D, and Ostermeier C. An improved method for fast, robust, and seamless integration of DNA fragments into multiple plasmids. Protein Expr Purif. 2006 Jan;45(1):66-71. DOI:10.1016/j.pep.2005.09.022 |
in-fusion
- Sleight SC, Bartley BA, Lieviant JA, and Sauro HM. In-Fusion BioBrick assembly and re-engineering. Nucleic Acids Res. 2010 May;38(8):2624-36. DOI:10.1093/nar/gkq179 |
In-Fusion biobrick
- Li MZ and Elledge SJ. MAGIC, an in vivo genetic method for the rapid construction of recombinant DNA molecules. Nat Genet. 2005 Mar;37(3):311-9. DOI:10.1038/ng1505 |
MAGIC, bacterial mating approach
-
j5 DNA Assembly Design Automation Software doi: 10.1021/sb2000116
- Tian J, Gong H, Sheng N, Zhou X, Gulari E, Gao X, and Church G. Accurate multiplex gene synthesis from programmable DNA microchips. Nature. 2004 Dec 23;432(7020):1050-4. DOI:10.1038/nature03151 |
- Gibson DG, Benders GA, Andrews-Pfannkoch C, Denisova EA, Baden-Tillson H, Zaveri J, Stockwell TB, Brownley A, Thomas DW, Algire MA, Merryman C, Young L, Noskov VN, Glass JI, Venter JC, Hutchison CA 3rd, and Smith HO. Complete chemical synthesis, assembly, and cloning of a Mycoplasma genitalium genome. Science. 2008 Feb 29;319(5867):1215-20. DOI:10.1126/science.1151721 |
- Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA 3rd, and Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009 May;6(5):343-5. DOI:10.1038/nmeth.1318 |
oligonucleotide assembly in vitro
- Gibson DG. Synthesis of DNA fragments in yeast by one-step assembly of overlapping oligonucleotides. Nucleic Acids Res. 2009 Nov;37(20):6984-90. DOI:10.1093/nar/gkp687 |
oligonucleotide assembly in yeast
- Gibson DG, Smith HO, Hutchison CA 3rd, Venter JC, and Merryman C. Chemical synthesis of the mouse mitochondrial genome. Nat Methods. 2010 Nov;7(11):901-3. DOI:10.1038/nmeth.1515 |
- Gibson DG. Enzymatic assembly of overlapping DNA fragments. Methods Enzymol. 2011;498:349-61. DOI:10.1016/B978-0-12-385120-8.00015-2 |
MIE paper
- Dymond JS, Richardson SM, Coombes CE, Babatz T, Muller H, Annaluru N, Blake WJ, Schwerzmann JW, Dai J, Lindstrom DL, Boeke AC, Gottschling DE, Chandrasegaran S, Bader JS, and Boeke JD. Synthetic chromosome arms function in yeast and generate phenotypic diversity by design. Nature. 2011 Sep 14;477(7365):471-6. DOI:10.1038/nature10403 |
- Hughes RA, Miklos AE, and Ellington AD. Gene synthesis: methods and applications. Methods Enzymol. 2011;498:277-309. DOI:10.1016/B978-0-12-385120-8.00012-7 |
Gene Synthesis Review
- Werner S, Engler C, Weber E, Gruetzner R, and Marillonnet S. Fast track assembly of multigene constructs using Golden Gate cloning and the MoClo system. Bioeng Bugs. 2012 Jan 1;3(1):38-43. DOI:10.4161/bbug.3.1.18223 |
MoClo
- Sarrion-Perdigones A, Falconi EE, Zandalinas SI, Juárez P, Fernández-del-Carmen A, Granell A, and Orzaez D. GoldenBraid: an iterative cloning system for standardized assembly of reusable genetic modules. PLoS One. 2011;6(7):e21622. DOI:10.1371/journal.pone.0021622 |
GoldenBraid
- Engler C, Kandzia R, and Marillonnet S. A one pot, one step, precision cloning method with high throughput capability. PLoS One. 2008;3(11):e3647. DOI:10.1371/journal.pone.0003647 |
GoldenGate
- Quan J and Tian J. Circular polymerase extension cloning of complex gene libraries and pathways. PLoS One. 2009 Jul 30;4(7):e6441. DOI:10.1371/journal.pone.0006441 |
CPEC
//T5 exonuclease recombination
- Li MZ and Elledge SJ. Harnessing homologous recombination in vitro to generate recombinant DNA via SLIC. Nat Methods. 2007 Mar;4(3):251-6. DOI:10.1038/nmeth1010 |
SLIC
- Li MZ and Elledge SJ. SLIC: a method for sequence- and ligation-independent cloning. Methods Mol Biol. 2012;852:51-9. DOI:10.1007/978-1-61779-564-0_5 |
SLIC
- Geu-Flores F, Nour-Eldin HH, Nielsen MT, and Halkier BA. USER fusion: a rapid and efficient method for simultaneous fusion and cloning of multiple PCR products. Nucleic Acids Res. 2007;35(7):e55. DOI:10.1093/nar/gkm106 |
USER
- Horton RM, Cai ZL, Ho SN, and Pease LR. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques. 1990 May;8(5):528-35.
SOEing
- Czar MJ, Anderson JC, Bader JS, and Peccoud J. Gene synthesis demystified. Trends Biotechnol. 2009 Feb;27(2):63-72. DOI:10.1016/j.tibtech.2008.10.007 |
review
- Aslanidis C, de Jong PJ, and Schmitz G. Minimal length requirement of the single-stranded tails for ligation-independent cloning (LIC) of PCR products. PCR Methods Appl. 1994 Dec;4(3):172-7. DOI:10.1101/gr.4.3.172 |
LIC
- Li C and Evans RM. Ligation independent cloning irrespective of restriction site compatibility. Nucleic Acids Res. 1997 Oct 15;25(20):4165-6. DOI:10.1093/nar/25.20.4165 |
LIC
- Angrand PO, Daigle N, van der Hoeven F, Schöler HR, and Stewart AF. Simplified generation of targeting constructs using ET recombination. Nucleic Acids Res. 1999 Sep 1;27(17):e16. DOI:10.1093/nar/27.17.e16 |
lambda Red recombinase
- Hartley JL, Temple GF, and Brasch MA. DNA cloning using in vitro site-specific recombination. Genome Res. 2000 Nov;10(11):1788-95. DOI:10.1101/gr.143000 |
Gateway lambda Int
- Khalil AM, Julius JA, and Bachant J. One step construction of PCR mutagenized libraries for genetic analysis by recombination cloning. Nucleic Acids Res. 2007;35(16):e104. DOI:10.1093/nar/gkm583 |
Gateway lambda Cre
- Larionov V, Kouprina N, Graves J, Chen XN, Korenberg JR, and Resnick MA. Specific cloning of human DNA as yeast artificial chromosomes by transformation-associated recombination. Proc Natl Acad Sci U S A. 1996 Jan 9;93(1):491-6. DOI:10.1073/pnas.93.1.491 |
Transformation-associated recombination (TAR) cloning
- Zhang Y, Werling U, and Edelmann W. SLiCE: a novel bacterial cell extract-based DNA cloning method. Nucleic Acids Res. 2012 Apr;40(8):e55. DOI:10.1093/nar/gkr1288 |
SLiCe