User:Susan Schultz/Notebook/Experimental Biological Chemistry/2011/09/07
 AuNP Synthesis AuNP Synthesis
|
|
|
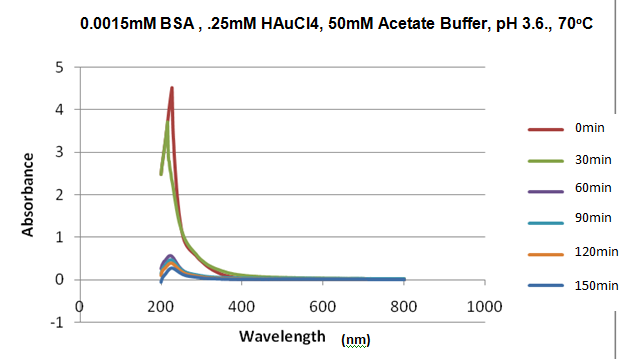
ObjectiveContinue investigating the pH dependance of the AuNP formation started on 8/31/11. Description1.Created a 10mL solution of 0.0015mM BSA and .25mM HAuCl4 mM in a 50mM Acetate Buffer, pH 3.6. 2. Created a 2nd 10mL solution using the same concentration of BSA and HAuCl4 in water. 2.Placed the solution in an oven at 80°C 3.Monitored each reaction using a UV-Vis Spectrometer between 200 and 800 nm every 30 minutes starting at time=0. DataA spectra was taken of a blank water and blank buffer solution, to be used as a reference. A spectra was then take of each reaction tube at time 0, 11:40, oven temp 79°C. It was monitored again at the following times. Removed 12:10, oven temp 74°C, replaced 12:20, oven temp 72°C. Removed at 12:50pm, oven temp 74°C, replaced 1:05pm, oven temp 72°C. Removed 1:35, oven temp 61°C, replaced 2:09, oven temp 68°C. Removed 2:38, oven temp 62°C, replaced 2:56, oven temp 66°C. Removed 3:36, oven temp 62°C. After the final measurement the samples were wrapped in aluminum foil and stored at room temperature.
Figure 1. UV-Vis Spectra taken of the reaction in acetate buffer at each time interval. Figure 2. The 550nm region of each spectra in figure 1. Figure 3. The absorbance at 550nm V. time for the reaction in the acetate buffer Figure 4. UV-Vis Spectra taken of the reaction in water at each time interval. Figure 5. 550nm region of figure 4 Figure 5. 550nm absorbance v. time for the reaction in water. NotesAt t90 dark purple strands were observed in each of the reaction tubes. It is hypothesized that these strands are the proteins coming our of solution as a result of the cooling which occurred during the extended periods of time the reactions were taken out of the oven. While the drop in absorbance in the 200-300nm range was first observed at t60, it is possible that it is a result of the material coming out of solution.
| |