User:Samantha R. White/Notebook/Biology 210 at AU
American University
The purpose of this lab is to examine the balance, complexities and interactions that exist in a transect of the campus of American University in Washington, DC.
February 24, 2016
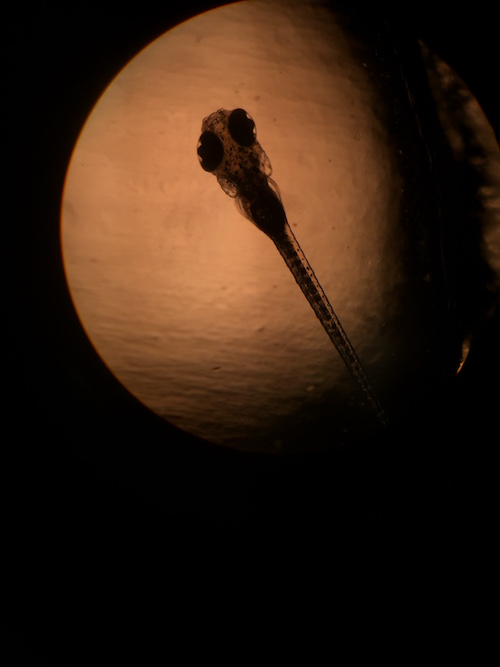
Figure 1: Image of a control solution zebrafish under a compound microscope. Eye measurement: 10 um; Body measurement: 80 um.
At seven days post fertilization, the fish embryos had hatched and showed development. The heartbeats were visible under the compound microscope. The epimephrine exposed fish showed a more rapid heart beat and sporadic limb twitching compared to the fish from the control solution who appeared still with relatively slower heartbeats. Both groups of fish were fed shrimp so they could to learn to eat and waste was removed from the wells. More of the epinephrine exposed fish had survived than the control solution fish. Two of the non surviving fish, one from each group, was anesthetized and then fixed in formaldehyde.
February 17, 2016
 Figure 1: This table shows the variation in characteristics by comparing starfish, frog, chick, and human embryos.
Figure 1: This table shows the variation in characteristics by comparing starfish, frog, chick, and human embryos.
Zebrafish Experiment: On day 1 of the experiment, a set of 20 zebrafish embryos were placed in 2 mL wells filled with purified water while another set of 20 were placed in 2 mL wells of an epinephrine solution. Epinephrine, also known as adrenaline, acts as a neurotransmitter in the "fight or flight" response in the brain and body. It is hypothesized that the fish embryos exposed to epinephrine in early development will display behavioral differences and possibly structural and functional differences in the body such as increased heart rate. The embryos were approximately 24 hours post-fertilization when the experiment was set up, and somewhere around the 4-8 somites stage. On day three of the experiment, 11 of the zebrafish in the control water dishes had not grown and 1 of the fish in the epinephrine solution had not grown. These were replaced with new embryos (24-hour post fertilization) and marked for identification.
February 10, 2016

Figure 1 represents data on the five invertebrates collected from Transect 2.
The size organism ranged from microscopic to inches in length. Several organisms of phylum arthropada were most frequently observed. These organisms floated to the top of the ethanol solution while they were not observed in the debris at the bottom of the sample, which had less abundant but more diverse arthropods.
Vertebrates and Niches:
Five vertebrates that are most likely be found to live within Transect 2 are:
- Chordata Aves Passeriformes Turdidae Turdus T. migratorius (American Robin)
- Chordata Aves Passeriformes Passeridae Passer P. domesticus (Sparrow)
- Chordata Mammalia Rodentia Sciuridae Sciurini (Tree Squirrel)
- Chordata Aves Passeriformes Cardinalidae Cardinalis C. Cardinalis (Cardinal)
- Chordata Aves Columbiformes Columbidae Zenaida Z. macroura (Mourning Dove)
Readily available water serves as a resource for organisms that exist in this plot of land. The biotic features include trees, bushes, and covered ground, which provide a habitat for various birds and squirrels to create nests in. The abundance of invertebrates serve as a source of nutrients for organisms to consume, even in the winter.

Figure 2 represents a food web of the organisms believed to be present in transect 2.
The organisms in this transect create a community by interacting in a sustainable way. The transect has a smaller carrying capacity for organisms that exist higher up in the food chain. It can support more microorganisms like bacteria and protists than can be counted, a limited range and number of plant life, and even fewer and less diverse populations of squirrels and birds at any one time.
February 2, 2016
Plant collection and observation:

This is a table to demonstrate the variation of plant life collected from the transect.

This is an image of the plant life collected from the transect.
Fungal observation:

This is a rendering of what could be viewed with the lens on the fungi sample.
January 27, 2016
Final hay infusion observation: The water within the hay infusion is cloudy and slightly brown with accumulation of particles near the decomposing leaves toward the bottom of the jar. The water line has receded approximately one inch from it's initial starting line due to evaporation. The water is cloudier just above the surface of the soil collected at the bottom, as if there are two separate layers. There is a film at the top of the jar consisting of residual milk powder. The surface appears milky and bubbly. The jar smells like decomposition of organic organisms and rotten milk and like feces.

Image 1: This table shows the serial dilution process and the concentrations of bacteria on each of the agar plates.
It was evident by looking at the plates that more growth took place on the plates that did not contain the tetracycline (an antimicrobial enzyme that works by interfering with the bacteria's ability to synthesize protein (http://www.chm.bris.ac.uk/motm/tetracycline/antimicr.htm)), and thus there were fewer colonies of bacteria on those specific plates, especially with those with a lower concentration of the serial dilutions of the initial bacteria solution. The presence of the antibiotic hindered, but did not prevent the growth of colonies on the plates.

Image 2: This table shows the characteristics of bacteria and categorizes them based on which plate they were observed from.
Most of the cells viewed under the microscope appeared to be rod-like in nature (with the possibility that one or more types could have been spiral shaped - not enough detail could be seen to determine) and were motile on the slide. Although the bacteria colonized on the plates, they appeared to be moving vigorously across the slide in random order.
Images 3-4: These images represent two of the four colonies observed within the data. These colonies were present on the plates that contained the tetracycline.
Image 5: This image represents one of the bacteria samples viewed under the microscope at 40x magnification. Notice the rod-like structures of the bacteria.
Materials and Methods: Use a loop tool, sterilized over the flame of a Bunsen burner, let it cool, and then use it to scrape a tiny amount of bacterial growth from the agar plate (from one colony). Place a drop of water onto a blank slide and then transfer the bacterial sample from the loop onto the water droplet in the slide. Use the heat fix method by passing the slide gently through the flame, (careful to not burn and damage the bacteria present on the slide) and then cover the bacteria in crystal violet dye and allow to sit for one minute. Rinse the stain with water. Cover the same slide with Gram's iodine for one minute and then rinse once again. Flood the slide with 95% alcohol for ten to twenty seconds, then rinse gently. Cover the slide with the safranin stain for 20-30 seconds and then rinse with water. Use a papertowel to blot excess water and allow to airdry. View under the microscope.


Images 7-8: Here are two examples of gram positive bacteria samples, grown from the untreated plates. Notice the purple-ish hue that indicated the presence of gram-negative bacteria.
SRW
January 20, 2016
The water within the hay infusion is cloudy and slightly brown with accumulation of particles near the decomposing leaves toward the bottom of the jar. The water is cloudier just above the surface of the soil collected at the bottom, as if there are two separate layers. There is a film at the top of the jar consisting of residual milk powder. The surface appears milky and bubbly. The jar smells like decomposition of organic organisms and rotten milk.
Samples were drawn from different locations within the hay infusion to increase the chance to see different protists. Samples were drawn from the top of the water near the milky film, from just above the bottom surface of the jar, and from just above the leaf debris.
Several species of protists were identified from each of the samples.
Surface of the jar:

Volvox; multicellular, motile, autotrophic algae
Above the leaf debris:

Didinium; unicellular, motile, heterotrophic protist

Vorticella; unicellular, motile, heterotrophic protist
Bottom of the jar:

Colpidium; unicellular, motile, heterotrophic protozoa

Paramecium; unicellular, motile, heterotrophic protest

Paranema; unicellular, motile, heterotrophic protest
January 13, 2016
This transect is located next to the amphitheater on the north side of campus. The ecosystem consists of a small rocky stream of water that flows from east to west. There are several large trees, bushes, and branchlings within the transect, as well as a variety of grasses and other vegetation on both sides of the stream. The soil is visible in parts and large flat rocks act as stepping stones from the south to north side of the transect.
Abiotic features:
- rocks
- stones
- water (in the stream)
- soil
- decomposing ground vegetation
Biotic features:
- bacteria
- protists
- trees
- bushes
- grasses and various plant life
- birds and squirrels
Below is the map that was drawn of the transect and a panoramic view (budding scientists as viewed are not included in biotic list).

Figure 1. hand-drawn diagram of basic transect features

Figure 2. panoramic view of transect as of Jan.13.2016
SW


