|
Objective
- Perform a Bradford Assay to determine the concentration of an unknown solution of Maltose Binding Protein (MBP)
Description
- Created 1.2 mL standards using 1, 2, 4, 6, 8, and 10 μg/mL BSA solutions in water with 200 μL Bradford Agent:
Blank - 0.000 μg/mL
Standard 1 (S1) - 0.833 μg/mL
Standard 2 (S2) - 1.667 μg/mL
Standard 3 (S3) - 3.333 μg/mL
Standard 4 (S4) - 5.000 μg/mL
Standard 5 (S5) - 6.667 μg/mL
Standard 6 (S6) - 8.333 μg/mL
- Created 1 mL samples of unknown MBP by 1/10, 1/100, and 1/1000 serial dilutions, and then added 200 μL bradford reagent.
- Obtained spectra from 200 - 800 nm of all standards and unknowns using UV-Vis Spectrophotometer (Shimadzu 2550).
Data
- Spectra of different concentrations of BSA.

- Calibration curve of the six standards at 595 nm. The equation was used to calculate the concentration of MBP.

- The absorbance of a 1/100 dilution of MBP at 280nm, used to calculate the concentration of MBP using the equation for the BSA standards at 595 nm.
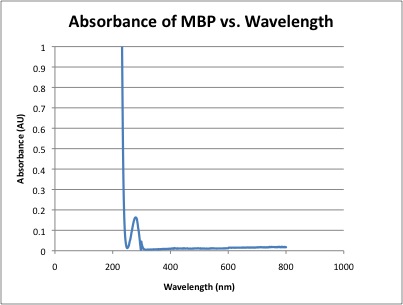
- After calculating the concentration of MBP, the molar absorptivity of MBP could be calculated for the entire spectrum.

Notes
- Corrected Absorbance of MBP at 280nm = 0.163
- Using equation for BSA/Bradford --> 0.163 = 0.0315x - 0.0205
x = concentration of MBP
x = 5.8253 μg/mL
- MBP is 1/10 dilution therefore 58.253 μg/mL is concentration of undiluted MBP.
58.253 μg/mL * 1000 mL / 1 L * 1 g / 1000000 μg * 1 mole MBP / 66776 g MBP = 8.723 * 10^-7 M
- Using beer's law molar absorptivity was calculated for the entire MBP spectrum.
A = εbc
ε = A / 8.723*10^-7
At 280nm ε = 196,033 L / cm * mole
- MBP dilutions used for calculating absorptivity were prepared again on 9/21, due to major inconsistencies in the data.
|
 Chem-571
Chem-571



