User:Dakota David/Notebook/Biology 210 at AU
'"February 10, 2014"'
Platyhelmenthes (acoelomate): est 5-10mm; cilia; observable eye; leads face first and squishes into itself; looks similar to a snake; mouth is on the side; it slides
Nematode (pseudocoelomate): wormy shaped; wiggles back and forth; squirms
Annelida, earthworms (coelomate): slow, rolling, writhing (although currently unmoving); they shrink along to move because they have more complex muscles
For procedure 2, we looked for 10 minutes becuase could not find any invertebrates from our Berlese funnel (on the top or bottom). We made an educated guess that perhaps the funnel was not warm enough for the insects to move down (because it was on the edge of the set-up not in the middle), so next time we would place it closer to the light. We also considered that it may have been too cold when we collected our sample (it was a particularly cold day for this time of year) and the location we selected within our transect may not have had enough insects in that area to begin with.
What kind of vertebrates would inhabit our transect?
1) Classification (phylum) - what biotic and abiotic factors would benefit them
2)
3)
4)
5)
Food web goes here
DD
'"February 3, 2014"'
Plant One


Angiosperm from a bush in the middle of our transect: xylem/phloem to transport nutrients and water; thorns/spiky petals; reproductive parts in middle of flower (anther/stigma); dicot (five petals)
Plant Two

Angiosperm- Black Oak- found in the soil under the bushes in the middle of our transect: broad leaf (5-6") with waxy cuticle; stomata evident; no visible reproductive parts; dicot; 5 lobes on leaf
Plant Three

Angiosperm- found under the bushes in the middle of our transect: appears to have parallel veins; long, narrow, grassy leaf (similar to onion grass); waxy cuticle; stomata unclear; stem has phloem/xylem; monocot
Plant Four

Angiosperm (cloverlike plant)- found alongside the bushes in the middle of our transect bordering with the grass: netted venation; green/purple leaves- soft, almost moss like; thick, waxy leaves; broad network of roots; dicot
Plant Five

Angiosperm (3 leaf clover)- found alongside the bushes in the middle of our transect bordering with the grass: small, green, soft, mossy plant; netted venation; small but broad leaves on top of a stem; broad network of roots; dicot
Fungi
Fungi sporangia are 3D circular structures which, when open, release spores for reproduction. We observed black bread mold (rhizopus sporangia zygots) from our tetracycline plate, an ascomycete. It is a ascomycete because it does not fit the basic requirements of a basidiomycete or a zygomycete. The mycelium and sporangia were clumped and tangled together in a mess of web.
We observed a black bread mold, an ascomycete. We observed the mycelium and sporangia, tangled together in a randomly arranged web. It is an ascomycete because it does not fulfill the characterists of a basidiomycete or a zygomycete.

A drawing of what we observed under the microscope.
DD
'"January 27, 2014"'
I hypothesize that the plates that do not have the tetracycline would have more colonies than those with the antibiotics.
Our hay infusions have become more clear, reduced in volume (half of where it had started), and there are no remaining sticks or grasses (except at the bottom which is now all sandy/sediment.
Table 1: 100-fold Serial Dilutions Results
Dilution Agar Colonies Counted Conversion Factor Colonies/mL
10^-3 nutrient 500+ x10^3 500,000
10^-5 nutrient 50 (+mold) x10^5 5,000,000
10^-7 nutrient 10 x10^7 100,000,000
10^-9 nutrient 1 x10^9 1,000,000,000
10^-3 nutrient/tet 100 x10^3 100,000
10^-5 nutrient/tet (mold/cannot count bacteria) x10^5 n/a
10^-7 nutrient/tet 1 x10^7 10,000,000
The plates that did not have tetracycline had more colonies of bacteria and of different varieties. The black colonies tended towards growing on the antibiotic-free plates, whereas the organge colonies were the only surviving type of bacterial colonies on the tetracycline plates suggesting that the antibiotic is more effective/targeted towards certain bacterias.
Tetracycline works by binding to microbial ribosomes, prevent the attachment of a specific type of tRNA, and thereby reducing reproduction in both gram-positive and-negative bacteria.
My data for this section is a little all over the place because I participated in a different lab section for this one, but also collected data from my actual group. All of the information I collected ais from the Prairie transect.
No tet (-3): Circular, raised, white, 1-3 microns, spirilium
No tet (-5): Irregular, darker in color-black, round cells (slightly more elongated), almost air-like bubbles present, convex, 1-2 microns

Drawing of sample at 40x magnification.
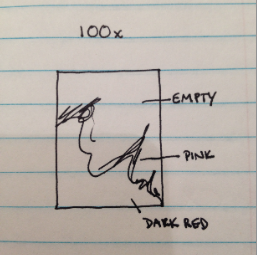
Drawing of sample at 100x magnification.
Tet (-6): Circular, convex, orange, looks almost like tetrads and wrinkled, clustered together, gram negative, pink, around 1 micron each

Drawing of sample at 40x magnification. Best guess: staph
DD
January 20, 2014
Procedure 1: How to use a dichotomous key
- We made a wet mount of the known organisms and identified a Euplotes around 175um long as well as an Amoeba proteus around 300um long.
Procedure 2: Hay Infusion Culture Observations
- Our Hay Infusion Culture had minimal smell, but it was murky and brown. It was clear enough to see sediment at the bottom that looked almost like sand. There were a few leftover stems of grass and sticks, as well as brown caking or mold on the sides of the jar. There was also a small dirt film beginning to grow on top of the liquid.
- We took a sample from the top of the liquid from the filmy area and a sample from the sediment. And identified these two organisms (we made multiple slides but were only able to identify amoebas):
- 
Figure 1
Figure 1: Colpidium ~50um

Figure 2
Figure 2: Amoeba ~5um
- Amoebas meet all the needs of life because it produces energy (consuming other organisms by surrounding it with its body as it does not have a stomach); it is a cell; it reproduces (cell division); and shares genetic information (genetic mutation across generations).
- If the hay infusion culture had been observed for another two months, I would predict that there would be more mold/film growth, and a wider variety of organisms available to view. However, that would vary because of the selective pressures that could possibly affect the organisms: such as food availability, space, and light.
Procedure 3: Preparing and Plating Serial Dilutions

Figure 1
Obtain four tubes of 10 mls sterile broth and label them 2, 4, 6, 7, as shown by "Figure 1".

Figure 2
Find four nutrient agar and four agar plus tetracycline plates. Label the tetracycline plates with "tet" and label one of each plate from the two groups 10-3, 10-5, 10-7, and 10-9, as shown by "Figure 2".

Figure 3
Swirl the Hay Infusion and take 100 microliters from the mix and add it to tube 2, as shown by "Figure 3". Swirl to mix well.

Figure 4
Take 100 microliters from tube 2 and inculcate tube 4. Repeat to make 10-6 and 10-8 dilutions, as shown by "Figure 4".

Figure 5
Take 100 microliters from the 10-2 (2) tube and place it on the surface of each of the plates labeled 10-3, as shown by "Figure 5". Repeat with tube 4 on 10-5, tube 6 on 10-7 and tube 8 on 10-9 plates.

Figure 6
Carefully spread the sample on the plate with the stick provided by the lab instructor, as shown by "Figure 6".
DD
'"January 13, 2014"'
Procedure 1: Prairie
- Our transect comprises the center of Freidheim Plaza, with a part of the grassy area, the bushes, and the stone benches.
- Biotic factors found: bushes, clover, leaves, florals, grass
- Abiotic factors found: stone, shells, soil/mulch wrappers, plastic guard rail, wrappers
DD