BME100 s2015:Group5 12pmL2
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | ||||||
OUR TEAM
LAB 2 WRITE-UPDescriptive StatisticsExperiment 1: Human Study Experiment 2: Rat Study
Graphing
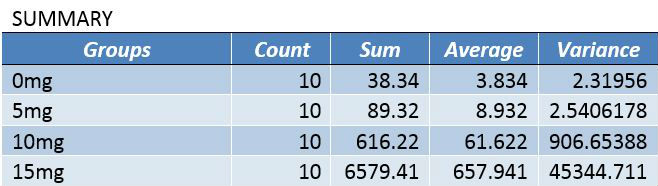
Inferential StatisticsAn ANOVA test was used in this experiment because of the P-Values that were concluded from the test, and because more than two groups of data are being compared for statistical significance. The P-Value shows the correlation between the dosages of LPS and the inflammation that was produced. The P-Value needed in the Bonferroni post-hoc tests is determined by dividing the alpha value for the P-test (.05) by the number of comparisons being done (six), which comes out to 0.0083333333. Because the T-Test value of each comparison in the Bonferroni correction is lower than the P-Value maximum determined through α/(number of comparisons), each group is statistically different from one another.
Summary/DiscussionIn the first experiment, 4 various LPS dosages were tested on human subjects. The four dosages given were 0mg, 5mg, 10mg, and 15mg. As the dosage was increased, there was a noticeable increase in the amount of inflammotin measured in the subjects. The smaller 2 dosages (0 mg and 5 mg) had insignificant levels in comparison to the higher dosages (10mg and 15 mg). Both t-tests and ANOVA were applied to the data to test the significance between the affect on inflammotin each of the dosages had. In conclusion, the ANOVA test proved that there was a significant change in data, making the results usable and reliable in proving the LPS has a positive effect on inflammotin levels. The second experiment was done on rats in 0mg and 10 mg doses of LPS. After the experiment was run, inflammotin activity did not numerically differ much between the 0 and 10mg dosages. Only a T-Test had to be run on the rat trials, as only two groups were being compared. The test showed that there was not a statistically significant change in the inflammotin levels between the two LPS dosages, as the T-Test's P-Value was below a 95% chance of being correct about significance. The human trials of LPS proved a positive relationship between the amount of LPS administered and the activity of inflammotin, while the rat trials did not show any significant relationship. This could be due to LPS working with human bodies in a way that differs from the way that it interacts with rats', as many compounds react with various species in a myriad of ways, due to differences in enzymatic behavior from species to species. Furthermore, the data from the rat trials may have lower certainty due to the limited number of trials run (10), as compared to the human trials, where 40 trials were run amongst 4 groups (with 10 in each group). The larger number of trials run in the human group allows for more validity, while the rat trials will require more trials in order to be treated with less skepticism. |
||||||