BISC219/F11: Lab 4
Lab 4: Forward Genetics Project-Complete Linkage Analysis; Start Mapping and Complementation Testing
Complete Linkage Testing
To determine in what chromosome or linkage group your dpy mutation is located, you first had to rule out the x chromosome. How did you do that? If you know that your unknown dpymutation is on an autosome, we want to know which one? To find out, you and your partner will first scan all 4 plates of progeny from your self-fertilization of the dpy/+; +/unc hermaphrodites that were created from crossing males that were heterozygous for our unknown dpy mutation with 4 different reference unc strains. If you did not get 4 successful reference crosses, see your instructor for help. Consult your cross diagrams to see what phenotypic ratio differences you expect between the progeny showing linkage of the two mutations and in progeny that have unlinked mutations.
TO DO TODAY
You and your partner should first scan all your plates to determine whether or not your crosses are scorable. If you have males or only phenotypicaly WT and Unc progeny, you should not try to analyze that plate for linkage. Why not? If you have no males and some dumpy progeny, you may score that plate in this linkage analysis. Look carefully at all the scorable plates and try to find double mutants. If you see no (or only very rare) double mutant (du/du) progeny on one of the plates, it indicates that your unknown dpy mutation is on the same chromosome or linkage group as that known unc mutation. Why? What might it mean if none of the plates of progeny lack double mutants? (Hint: there are 5 autosomes and we only tested 4 of them for linkage.) Since we know on which autosome each of the reference unc mutations are located, you should be able to figure out on which chromosome your unknown dpy mutation is found.
To confirm these findings, please score and record the number of wild type, Dpy, Unc and Dpy Unc mutants. We will do this scoring as a class (and course) and compile our data so you and your partner will only be asked to score 50 worms from one of the strains tested. Your instructor will assign the reference strain that you should score. Make sure that you record these data on the course spreadsheet before you leave lab.
Since your dpy mutation can be in a linkage group with only one of the unc reference strains, the others should show evidence of independent assortment of the dpy and unc alleles. What will independent segregation score like in these progeny? Why? What is number of the autosome on which your unknown dpy mutation is found?
| Chromosome | Strain | Phenotype |
|---|---|---|
| Chromosome 1 | unc-13 | coiler |
| Chromosome 2 | unc-52 | immobile |
| Chromosome 3 | unc-32 | coiler |
| Chromosome 4 | unc-17 | coiler |
Mapping a Mutation to a Specific Location on a Chromosome and Gene
Assuming that you have determined the linkage group on which your dpy mutation resides, we will continue working with that strain only. You now suspect that the two mutations (the dpy mutation of interest and the reference mutation unc) are on a particular autosome. Use Worm Base http://www.wormbase.org to look up the reference unc mutation and note its location on the chromosome. To begin to calculate distance between this marker and your dpy mutation of interest, you will separate 6 unc single mutants to 6 individual plates. Although most of these Unc individuals do not carry a defective copy of the dpy gene (genotype (+ u/+ u); however, we hope that we will pick at least a couple of wild type Unc individuals that are heterozygous for the dpy mutation (d u/+ u). Why aren't they all (+ u/+ u) ?
Finding double mutants (du/du)
To Do Today:
- Using only the linkage analysis plate with the linked unc strain, separate 5 Unc mutants to 5 individual plates (This plate should also have Dpy mutants!)
- Label each of these plates with "Mapping 1" and your initials and the date with your PURPLE Sharpie.
- Incubate the plates at 23°C for 3 days
3 days later
- Screen your 5 plates for double mutants (Dpy mutants that either do not move or coil).
- Pick 3 such putative double mutants to separate plates to allow them to self fertilize. If they are truly double mutants then all of their progeny should be double mutant as well and the progeny will be used for the next cross.
- Label each of these plates with "Mapping 2", the genotype d u/d u and your initials and the date with your PURPLE Sharpie.
- Incubate the plates at 23°C until next lab period.
Complementation
In order to find out if a mutation under study in a forward genetics project is likely to be a newly discovered mutation or is,perhaps, in a previously characterized gene, we will perform a complementation analysis. If our mutation and gene has been previously characterized, this complementation analysis might tell us the name of our gene of interest. It is not unusual to have series of mutations that confer similar phenotypes and also map to a identical or similar location on a chromosome. Complementation testing can determine if two mutations are allelic (that is, in the same gene) or non-allelic (in different genes but both causing the same phenotype). This is done by crossing a mutant with a series of reference strains. In our case, we will use several different reference mutant strains. All have a Dpy phenotype, but in each strain the gene responsible for the dumpy defect has been located to a different known region of a chromosome.
If the mutations (your unknown's and that of a reference strain) are allelic (in the same gene but on different sister chromatids) there should be no complementation, meaning that the mutations can not "rescue" each other because you do not have a normal copy of the gene causing the mutant phenotype; thus, you observe the Dpy phenotype when the two strains combine.
If the mutations are non-allelic (in different genes) there should be complementation, meaning they "rescue" each other: because there is one normal copy of each defective recessive gene, the product of the two parental homozygous dpy mutants can show a wild type phenotype.
Complementation analysis can be evaluated this way only when you are working with recessive mutations.
For more information about complementation, see your Genetics textbook or Wikipedia Definition.
The specifics of strain construction for complementation analysis vary depending on the experimental organism; however, the basic strategy in all cases is to construct a double heterozygote organism and then to examine the phenotype of this organism. Remember that a wild-type phenotype indicates that the two mutations complement one another (cancel each other out) and are therefore in different genes. Conversely, a mutant phenotype suggests the mutations are allelic to one another (that is, they fail to complement).
Over the next two weeks we will construct different double heterozygotes containing your dumpy mutation of unknown location (dpy-u) and dumpy mutations of known location (dpy-k) on the same chromosome as your unknown dpy mutation as follows:
First obtain heterozygotes for your dpy mutation of unknown location through
Cross #1: unknown Dpy hermaphodites (dpy-u/dpy-u) x N2 males (+/+) yields dpy-u/+ progeny
Then use those heterozygous males resulting from Cross #1 in the next cross:
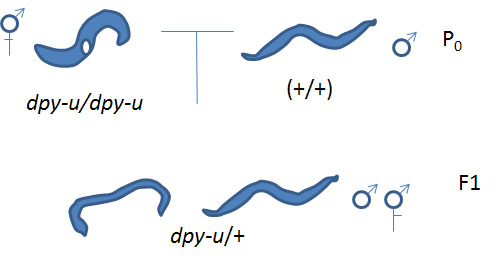
Cross #2: dpy-u/+ males x known dpy hermaphrodites (dpy-k/dpy-k) yields some dpy-u +/+ dpy-k progeny. [Note: we need to use heterozygote males for our unknown dpy mutation (dpy-u/+) because homozygote dumpy males do not mate properly]
Available Dpy strains:
| Chromosome 1 | Chromosome 2 | Chromosome 3 | Chromsome 4 |
|---|---|---|---|
The phenotype of the double heterozygote is then scored.
Keep in mind that, in this experiment, you will determine the allelic counterparts of your “unknown” Dpy mutation by placing it (your unknown mutation) in trans with mutations in different Dpy genes that have been previously mapped (i.e., known mutations) to your chromosome of interest. Our goal is to determine whether or not our “unknown” Dpy mutations are allelic to any of the known mutations.
To Do Today
- Determine which dpy mutations are located on the same chromosome as your unknown.
- See which of these mutant strains we have available to determine how many crosses you will perform.
- Set up Cross #1: Cross your unknown Dpy hermaphrodites with N2 males [(dpy-u/dpy-u) x N2 males (+/+)] by placing three to five L4 Dpy's on a new plate with 3-4 N2 males. You will be picking your own wild type males from a mixed population of worms so be sure to transfer your males to a transfer plate before adding to the crosses. It is essential that the ONLY wild-type animals present on this plate are males.(There should be no wild type hermaphrodites because you desire +/dpy-u males from this plate for the next cross, and not the +/+ males that would result of wild-type hermaphrodites were included on the plate). Do this in duplicate if there are enough worms. (Check with your instructor about that.)
- Label your plate(s) with your initials and the date and the genotype dpy/dpy (H) X +/+ (M) with your ORANGE Sharpie.
Incubate the worms at 23°C for 3-4 days.
TO DO IN 3 DAYS
You will now set up Complementation Cross #2: Pick 3-5 males from the cross plate initiated in lab this week onto three new mating plates (remember these are heterozygous for your dpy mutation so they are phenotypically wild type but they carry dpy-u (dpy-u/+). Again, it is essential that only males be transferred onto these plates. Add three L4’s from each of the 3 known dpy reference strains to the mating plates with the males.
Available Dpy strains:
| Chromosome 1 | Chromosome 2 | Chromosome 3 | Chromsome 4 |
|---|---|---|---|
Label your plates, using your Orange Sharpie, with your initials and the date and the cross dpy/+ (M) X dpy-k (replacing k with the gene number of each of the reference strains)/dpy-k.
Incubate the worms at 23°C for 3-4days.
Assignment
Remember to check the Assignment section of the wiki for instructions about the graded assignment due in the next lab and check the Weekly Calendar for other work to accomplish before the next lab.
