Artificial Trachea by Megan Greiner, Ian Costello, and Chris Carr
Background

The trachea, also known as the windpipe, is the passage way that leads air to your lungs (figure 1). It is made up of cartilage, muscle and connective tissue. Tracheas are often around four inches long and comprised of approximately twenty cartilage rings. On the inside lining of the trachea there is a mucus membrane that is able to catch harmful airborne particles and bacteria before it reaches your lungs. Swallowing and sneezing are ways of removing these particles [1].
Breathing involves two main steps; oxygen enters the body when you breathe in and carbon dioxide leaves the body when you exhale. When someone takes a breath, oxygen goes through the nose and mouth down their trachea. From here the trachea splits up into two bronchi tubes that lead to the left and right lung. Next the oxygen travels through the smaller branches, bronchioles, to millions of small alveoli located throughout the lungs. Alveoli are small sacs in the lungs that allow for the exchange of gases with the blood stream. Here oxygen is exchanged with carbon dioxide. The red blood cells deliver the oxygen to the rest of the body while the carbon dioxide is expelled out the same way the oxygen came in. To avoid food and drink from entering the trachea and lungs, a flap, known as the epiglottis, closes while swallowing [2].
Current Tracheal Health Issues
Many diseases can affect the trachea and diagnosis may be complicated by generalized symptoms such as coughing, wheezing, and shortness of breath. As a result, these diseases can become fatal if not treated or detected fast enough. Computed tomography (commonly referred to as CT) is often used to diagnose trachea abnormalities caused from disease or illness. Diseases of the trachea that can benefit from a trachea replacement are [3]:
Tracheal Cancer: Although rare, tracheal cancer is very fatal if undetected right away (5% survival rate). Tracheal neoplasms are lumps or growths, which can result in malignant tumors (figure 2). There are two types of neoplasms, one of which is benign while the other is malignant. Benign neoplasms would most likely be surgically removed while malignant neoplasms would benefit from a full tracheal replacement. A majority of tracheal tumors are a result of these malignant neoplasms, which is most commonly squamous cell carcinoma (the second most common form of non-melanoma skin cancer, common in head/neck cancers) [3,4].

Tuberculosis: An infectious disease, which is caused by various strains of mycobacteria. It is an infection that attacks and severely damages the lungs. Tuberculosis can cause strain to not only the lungs from excessive violent coughing, but also to the trachea. Tuberculosis can result in a collapse of the tracheal branch of the windpipe as well as tracheal stenosis (a narrowing of the windpipe). Both of these conditions can be cause for a tracheal transplant. While tuberculosis is not extremely common in the United States, in 2010, 1.5 million deaths were attributed to tuberculosis globally [5,6].
Tracheal stricture (stenosis): An abnormal narrowing of the trachea, which is usually caused because of trauma to the neck. It can be cured by temporary enlargement using tracheal dilation (lasts for 3 days-6 months), resection (cut and sewn back together to enlarge), or a full tracheal replacement [3].
Tracheomalacia: A weakness of the tracheal walls, which leads to collapsed airways. This is common in children with cartilage deficiencies and can also be caused in adults due to injury or smoking. This typically is not serious enough for surgery but in extreme cases replacement of the trachea may be necessary [1,3].
Tracheoesophageal fistula: When a channel forms between your esophagus and trachea. The esophagus is responsible for transport of food and water. When a channel forms between the esophagus and trachea, food and water may travel down the windpipe possibly causing damage to the lungs [1].
History
1981 - Start of skin tissue engineering of epithelial cells [7]
2000 - Artificial trachea trials and follow-ups on dogs [8]
2008 - First cadaver trachea transplant seeded with patients own cells by Macchiarini [9,10]
2010 - Macchiarini performed first artificial trachea implanted with patient's stem cells [9,10]
2012 - First child to receive a stem cell grown trachea (donor trachea) by Macchiarini [11]
2013- Laryngo-Tracheal Tissue-Engineered Transplantation Clinical Trial [12]
2014- Paolo Macchiarini accused of medical misconduct [13, 14]
2015- Clinical Study: 3D Printed Tracheobronchial Splints for Pediatric Patients [15]
Types of Tracheal Replacements and Associated Problems
Necessities for a well-designed trachea replacement [16]:
1. Maintain airway during breathing
2. Support the windpipe
3. Chondrocytes and epithelial cells
Acellular artificial prosthetic: An artificial prosthetic usually made from polymer that does not contain cells. New connective tissue or blood vessels can form on the incision or wound site (tissue granulation), there can be shifting of the implant, scar tissue, and narrowing of an artery or the airway (restenosis) [16].
Autograft: Tissue taken from a different location in the patient’s own body. It has limited ability, mechanical properties can be inadequate for patient needs, and the implant can change post operation, which can cause airway blockage [16].
Native or decellularized allograft: Tissue taken from a donor and replaces donor cells with patient cells. There is risk of rejection and transmitted diseases [16].
Autologous tissue engineered construct: A construct made with the patient’s cells. It requires specific design parameters to guide cell behavior and new tissue growth [16].
Current Technology
Cadaver Implant
Since 2008, there have been ten trachea implants using tracheas from cadavers. The tracheas are harvested, and chemically treated to remove all original cells. Once the cells have been removed from the donor trachea, it is essentially used as a scaffold. Stem cells are taken from the patient’s bone marrow ( Bone Marrow Transplants, by Erinn Dandley, Max Nowak and Jean Smith ) and are seeded onto the scaffold trachea. The scaffold is inserted into a bioreactor where the patient’s stem cells and certain transcription factors are added to induce specific differentiation on the scaffold. Once the cells have differentiated to a certain point, the trachea is implanted into the patient where the body takes over and induces more cell differentiation and proliferation. A disadvantage to using a donor trachea for a transplant is that the amount of cadaver tracheas available is limited; additionally, the donor tissue may not be compatible with the patient in terms of size, immune response, etc. [7, 17].
Tissue Engineered Implant
The first artificial trachea surgery was led by Dr. Macchiarini. The scaffold was made from plastic nano-fibers and seeded with stem cells from the patient’s bone marrow. Similarly to the cadaver transplant, it is placed in a bioreactor in order for the cells to grow. In order to create an artificial trachea the patient is first imaged by a CT scan. This is so the tissue engineers can make a scaffold that fits perfectly within the patient’s body. The scaffold is then made from nano-sized PET (polyethylene terephthalate) fibers using these specifications. Next, it is seeded with stem cells from the patient’s bone marrow by placing it in a bioreactor with transcription factors. These help force the stem cells to differentiate into trachea-specific cells. These cells then grow and divide to produce cartilage around the scaffold after two to three days. Finally, the artificial trachea is sutured to the patient’s throat and lungs. The estimated cost of this entire surgery is about $450,000. The advantage to using an artificial trachea compared to using a cadaver is that it is custom fit to the patient and no anti-rejection drugs are required [7].

There is low risk of rejection due to using the patient’s own stem cells. However, with any type of foreign object in the human body there is always some chance of rejection, so the patient should watch for any similar symptoms. Along with this, it seems that there is a long recovery with a transplanted trachea. One patient was doing “well” after four months and was able to walk 500 meters without stopping [7,8]. With this in mind, the artificial trachea will be more beneficial and have a higher potential to save lives since it can be custom made. However, the first child to be given a new trachea used a donor trachea combined with his own stem cells. In this procedure they continued to add growth factors after it was introduced to promote growth and save time from growing the cells outside the body. In the end he was taken off the anti-rejection drugs and the trachea has even grown 11cm (18 months after the procedure) [8,11].
Types of Cells and Polymers Used
Mesenchymal Stem Cells
People have experimented with scaffold free systems to avoid the potential problems associated with a scaffold. Attempts have been made using chondrocytes that have fully matured (differentiated). This poses complications as well because the cells need to come from a donor and cartilage tissue has a small amount of cells, so a good amount of tissue would have to be donated. The donor site can be painful and lose function. Tissue donation to retrieve these cells could also involve a surgery, which poses surgical risks. An alternative using mesenchymal stem cells ( Mesenchymal Stem Cells in Tissue Engineering by Chelsea Orefice ) has been researched because they can be taken from a patient with less risk and are easier to expand in vitro than differentiated cells. They also can be differentiated into multiple connective tissue phenotypes needed for an artificial trachea replacement [16].
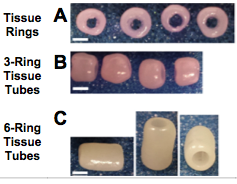
In 2015, a group researched how to make self assembled mesenchymal cell constructs. The researchers had to create rings from human mesenchymal stem cells (figure 4) from bone marrow. The rings were created with agarose culture wells where the stem cells differentiated into cartilage tissue. After two days the rings were stacked together to form tubes. Growth factors for cartilage development were delivered with microspheres to the cartilage rings and tubes to boost the growth of new cartilage tissue formation and increase mechanical properties. It was concluded that the mesenchymal stem cell constructs had at least the same biomechanic properties, if not better properties, than those of a natural rat trachea; therefore, suggesting that this method could be used in animal models [16].
Porcine Cartilage Derived Substance
Previous work has been done with using fibrin/hyaluronic acid composite gel to create artificial tracheas and proved to be biocompatible, however did not create a lot of new cartilage and lacked chondrocyte survival. A scaffold that promoted new cartilage tissue growth was the goal, so in 2014 porcine cartilage derived substance (PCS) was used as a scaffold because it is very porous. Also, the rate at which PCS degrades can be easily manipulated. Porcine cartilage was taken from pig legs to use as a scaffold for the artificial trachea. The cartilage was washed several times and decellularized with a buffer solution of Tris-HCl. After sterilization and incubation, the porcine cartilage scaffold was implanted with chondrocytes taken from knee cartilage in rabbit models. The scaffold was culture for 7 weeks before implanted into rabbit models. It was found that the PCS scaffold allowed for 90% chondrocyte survival, survival of growth factors, and was favorable for formation and survival of ciliated epithelial cells. The drawback to using PCS is that it lacks mechanical strength and takes a long period of time to gain the strength needed for a transplant [18].

Collagen Sponge and Polypropylene Scaffolds
PLA sheets with PGA mesh ( PLA, PGA, and PLGA as biomaterials, by Colton Kenny ) were initially used to create artificial tracheas used in rabbits. This was done with a basic fibroblast growth factor, but stents were required post operation to avoid tracheal collapse and a blocked airway. It also caused an immune response and inflammation. In 2010, a hybrid structure was used with PLGA and collagen solution to create a more porous site, however the collagen solution was not ideal because it did not favor epithelial growth [19].
In 2015, a different group did a study where artificial tracheas were developed using collagen sponges at different concentrations and polypropylene scaffolding (also with rabbits). It was found that the lower percentages (0.5% and 1% collagen solution) proved to be more porous and therefore allowed for more epithelial tissue growth. Epithelial tissue grew within 14 days with the porous sponge, however with a nonporous sponge (1.5% collagen solution), it took longer. Also, it was proven that a porous sponge promotes mesenchymal cells to invade the area and therefore the healing process becomes more rapid [20].
Synthetic versus Natural Scaffolds
The most common type of natural scaffold is a donor trachea that has the donor cells removed and is washed multiple times. Then it becomes cellularized again with the patient cells. This involves physical treatments: freezing, thawing, agitation. It also involves chemical treatments, such as trypsin, to break cell membranes when decelluarizing. Unfortunately, this is not a permanent solution as stenting is necessary after a period of time post operation [21].
Synthetic scaffolds are easier to work with because they can be modified specifically for the patient. At first solid materials were used like stainless steel or silicon. Since a porous structure has proven crucial, polymers like polycarbonate urethane or polyhedral oligomeric silsequioxane are used. They are strong enough to provide structural support, but also are softer and promote proliferation of cells. These polymers can also be manipulated in terms of porosity, size, and strength depending on the patient’s needs [21].
Clinical Trials
Laryngo-Tracheal Artificial Trachea Implants
In 2013, a clinical trial was proposed where thoracic surgeon, Paolo Macchiarini, was the principal investigator. The clinical trial involved replacing the trachea with a synthetic scaffold (polyurethane or polyethylene) seeded with autologous mononuclear cells. The targeted patients were those who had terminal tracheal diseases. This procedure would be a solution when resection of the diseased tissue was not possible. Specifically, the scaffold would cultivate for about 48-72 hours in a bioreactor where growth factors would be injected into the scaffold at the beginning and end of cultivation. This scaffold would then replace the diseased or impaired trachea [12].
The criteria to be a patient in the trial was: more than 60% of the trachea is diseased, patients have received all other possible treatments, there were no conditions or factors that would lead to harm of patient (contraindication), patient had no regional or micro metastasis, granted permission from Institutional Review Board, Ethics Board, and National Transplant Coordinators, written consent from the patient, and the patient has normal psychological behavior. Patients were denied clinical trial treatment if they had metastatic malignancies or a psychological condition [12].
Postoperative measures were set up to evaluate patient outcome. The safety of the trachea itself would be measured by how many times complications occurred a year after the operation. The number of patients who survived a year after the operation and the number of patients who were disease free would also be taken into account to conclude if the artificial trachea improved patient conditions. There were six participants in the trial that ranged from 28 to 41 years of age. Two of the participants were female and the remaining four were male [12].
Three days prior to transplantation the bone marrow mononuclear cells are taken from the patient, isolated from red blood cells, and suspended in cell culture. The resulting product is tested to determine cell type for the patient. For the two days prior to the transplantation, the patient receives injections to increase cell mobilization. The artificial scaffold incubates for 96 hours (48 hours while being created and 48 hours before the operation) and is removed for implantation. According to the available information, six patients were analyzed for the safety of the stem cell artificial tracheal scaffold and zero of them appeared to have safety concerns. All six of the participants survived a year after the operation and they were all considered to be free of disease [12].
3D Printed Tracheobronchial Splints

About 43% post pediatric tracheostomy patients go into respiratory arrest per year due to tube obstruction. It has been shown that overall, if a pediatric patient can survive the initial 36 months of tracheobronchomalacia then the disease will fix itself. There was a clinical study completed in 2015, where three pediatric patients with tracheobronchomalacia were treated with a 3D printed tracheobronchial splint to assist with the growth of their airway during the crucial time period of the first 36 months [15].
The 3D printed devices were made specifically for each patient by using computed tomography (CT) images and CAD software. This would give specific dimensions and geometry of each patient’s trachea. The splints were printed with polycaprolactone, which can stay viable in the body for up to two to three years before absorption into the pre-existing tissue. The time it took from initial patient evaluation to the finished product being printed was on average about 3 days for the three patients ranging from 2-5 days. The 3D printed device can be seen in figure 5. The devices would be able to withstand growth of the pediatric patients, assist with the growth, and prevent external pressure on their airway. The splints were placed around the diseased or injured airway with polypropylene sutures [15].
Patient one had no complications during or after the procedure. Three weeks post operation; the patient was completely off ventilator support and discharged. At about 3 years post operation, the splint started to degrade; nonetheless the patient is still without tracheobronchomalacia symptoms. Patient two was taken off ventilator support fifteen weeks after the operation and was discharged from the hospital for the first time in his life. Patient three did not have any complications during the procedure or after specifically because of the device. However, the patient still remains on ventilation support most likely because his condition is worse than what the device was designed to resolve. Overall, all three patients had instantaneous improvements with their condition due to the 3D printed splints [15].
Controversy

Recently there has been a lot of media hype on a popular figure who has been spear heading the artificial trachea movement, Paolo Macchiarini. Macchiarini is a thoracic surgeon at Karolinska Instituet in Sweden and has performed trachea transplants on eighteen patients using cadaver tracheas and stem cells. He performed the first of ten artificial trachea surgeries in 2008 using a biological scaffold. There have been seven more surgeries after 2011 that have involved electrospun scaffolds [22]. Investigations have been happening regarding several accusations, such as fraudulent academic credentials, unethical decision making, and mostly transgression on several of his published papers regarding patient procedures and outcomes. For example, the papers do not fully disclose the postoperative issues patients have had to deal with, such as a follow up procedure to receive a stent. Moreover, it has been stated that Macchiarini has not fully disclosed all possible risks to patients and was reported by Macchiarini’s colleagues that two patients did not have informed consent forms on file [13, 14].
Out of the eight synthetic trachea transplants performed, only two still remain alive. The six patients that have since died only lived for three months to two and a half years after the operation. Macchiarini has claimed that all of these deaths are not linked to the trachea transplant and have been due to instances like alcohol or multiple system organ failure. Ultimately Macchiarini emphasizes that this is a risky procedure and is still experimental, but the question remains if he disclosed this to his patients in a truthful manner [14].
Throughout this scandal, Macchiarini was found guilty of getting involved with an American television producer working on a documentary about the work he was doing with artificial tracheas. He was caught in many lies with this woman all to ensure that his work would be shed in a positive light. This has only added fuel to the controversy [13]. There are mixed views on the surgeon, where some claim, “from a professional standpoint, this guy’s the real deal” [13] and others say that what he has done is “alarming” [23]. Even so, the Karolinska Instituet informed reporters that Paolo Macchiarini’s contract will not be renewed come November 2016 [23].
Future Work
As of now there have only been clinical studies, mainly on animals, involving artificial trachea transplants. Currently, there is only one method that is accepted for clinical use, which is a 3D extra cellular matrix scaffold that does not inhibit an immune response and still demonstrates the same qualities of a natural trachea [24]. Thus far, 3D printing and synthetic scaffolds show promise; however, surgical procedures need to be developed and the ideal biomaterials for scaffolds need to be determined [25].
References
[1] "The Trachea." WebMD. WebMD, n.d. Web. 16 Apr. 2013. <http://www.webmd.com/lung/picture-of-the-trachea>.
[2] "How Does the Breathing Process Work?" EHow. N.p., n.d. Web. 16 Apr. 2013. <http://www.ehow.com/how-does_4565395_breathing-process-work.html>.
[3] Kwong, Stephen, MD, Nestor Muller, MD, and Roberta Miller, MD. "Diseases of the Trachea and Main-Stem Bronchi: Correlation of CT with Pathologic Findings." (n.d.): n. pag. Radiographics. Radiographics.rsna.com, 20 Mar. 1992. Web. 16 Apr. 2013. <http://radiographics.rsna.com/content/12/4/645.full.pdf>.
[4] Daley, Brian. "Tracheal Tumors ." Tracheal Tumors. Emedicine.medscape.com, 19 Aug. 2011. Web. 16 Apr. 2013. <http://emedicine.medscape.com/article/425904-overview>.
[5] ] "Signs & Symptoms of Tuberculosis." - Tuberculosis (TB). Healthcommunities.com, 1 June 2000. Web. 16 Apr. 2013. <http://www.healthcommunities.com/tuberculosis/symptoms.shtml>.
[6] Silverman, Gertrude. "Tuberculosis of the Trachea and Major Bronchi." Chest Journal (n.d.): n. pag. Chestnet.org. 1945. Web. 16 Apr. 2013. <http://journal.publications.chestnet.org/article.aspx?articleid=1051893>.
[7] Fountain, Henry. "Synthetic Windpipe Is Used To Replace Cancerous One." The New York Times. The New York Times, 12 Jan. 2012. Web. 16 Apr. 2013. <http://www.nytimes.com/2012/01/13/health/research/surgeons-transplant-synthetic-trachea-in-baltimore-man.html?_r=5>.
[8] Nakamura, T. "Artificial Trachea and Long Term Follow-up in Carinal Reconstruction in Dogs." Int J Artif Organs (2000): n. pag. PubMed. Web. 16 Apr. 2013. <http://www.ncbi.nlm.nih.gov/pubmed/11075903>.
[9] Murray, Peter. "In Medical First Doctors Implant Lab Grown Synthetic Trachea Into Patient."Singularity Hub. N.p., 9 July 2011. Web. 16 Apr. 2013. <http://singularityhub.com/2011/07/09/in-medical-first-doctors-implant-lab-grown-synthetic-trachea-into-patient/>.
[10] Walker, Andrea. "Abingdon Man First in the U.S. to Get Synthetic Trachea Transplant."Baltimore Sun. Baltimore Sun, 13 Jan. 2012. Web. 16 Apr. 2013. <http://articles.baltimoresun.com/2012-01-13/health/bs-hs-trachea-transplant-20120112_1_tumor-windpipe-trachea>.
[11] Salahi, Lara. "Stunning Recovery for First Child to Get Stem Cell Trachea." ABC News. ABC News Network, 26 July 2012. Web. 16 Apr. 2013. <http://abcnews.go.com/Health/stunning-recovery-child-stem-cell-trachea/story?id=16858771>.
[12] Kuban State Medical University. Laryngo-Tracheal Tissue-Engineered Clinical Transplantation. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [updated 2015 May 18; cited 2016 Feb 22]. Available from: https://clinicaltrials.gov/ct2/show/study/NCT01997437 Identifier: NCT01997437 <https://clinicaltrials.gov/ct2/show/study/NCT01997437?show_desc=Y§=X43a987016&view=record>
[13] Ballingall, Alex. "Prominent Surgeon Paolo Macchiarini Cut down by Controversy | Toronto Star." Thestar.com. Toronto Star, 19 Feb. 2016. Web. 22 Feb. 2016. <http://www.thestar.com/life/health_wellness/2016/02/19/prominent-surgeon-paolo-macchiarini-cut-down-by-controversy.html>
[14] Cyranoski, David. "Investigations Launched into Artificial Tracheas." Nature.com. Nature Publishing Group, 28 Nov. 2014. Web. 22 Feb. 2016. <http://www.nature.com/news/investigations-launched-into-artificial-tracheas-1.16431>
[15] Morrison, Robert J., et al. "Mitigation of tracheobronchomalacia with 3D-printed personalized medical devices in pediatric patients." Science translational medicine 7.285 (2015): 285ra64-285ra64. PMID 25925683
[16] Dikina, Anna D., et al. "Engineered cartilaginous tubes for tracheal tissue replacement via self-assembly and fusion of human mesenchymal stem cell constructs." Biomaterials 52 (2015): 452-462. PMID 25818451
[17] "Cancer Patient Pioneers First Stem Cell Trachea Transplant - HSCC Online Provide News Spotlight about Stem Cell and Medicine - HSCC Online Provide News Spotlight about Stem Cell and Medicine." Hongkong Stem Cell Centre -Hong Kong Stem Cell Centre, Jan. 2012. Web. 12 Apr. 2012. <http://www.hongkongstemcell.com/c/e_information_244.php>.
[18] Shin, Yoo Seob, et al. "Tissue-engineered tracheal reconstruction using chondrocyte seeded on a porcine cartilage-derived substance scaffold." International journal of pediatric otorhinolaryngology 78.1 (2014): 32-38. PMID 24280440
[19] Tatekawa, Yukihiro, et al. "Tracheal defect repair using a PLGA–collagen hybrid scaffold reinforced by a copolymer stent with bFGF-impregnated gelatin hydrogel." Pediatric surgery international 26.6 (2010): 575-580. PMID 20425118
[20] Nakaegawa, Yuta, et al. "Effect of Structural Differences in Collagen Sponge Scaffolds on Tracheal Epithelium Regeneration." Annals of Otology, Rhinology & Laryngology (2015): 0003489415599991. PMID 26276144
[21] Crowley, Claire, Martin Birchall, and Alexander M. Seifalian. "Trachea transplantation: from laboratory to patient." Journal of tissue engineering and regenerative medicine 9.4 (2015): 357-367. PMID 26052583
[22] Jungebluth, Philipp, et al. "Tracheal tissue engineering in rats." Nature protocols 9.9 (2014): 2164-2179. PMID 25122525
[23] Vogel, Gretchen. "Karolinska Institute Has 'lost Confidence' in Paolo Macchiarini, Says It Won't Renew His Contract." Karolinska Institute Has 'lost Confidence' in Paolo Macchiarini, Says It Won't Renew His Contract. Science Magazine, 04 Feb. 2016. Web. 22 Feb. 2016. <http://www.sciencemag.org/news/2016/02/karolinska-institute-has-lost-confidence-paolo-macchiarini-says-it-wont-renew-his>
[24] Peloso, Andrea, et al. "Current achievements and future perspectives in whole-organ bioengineering." Stem cell research & therapy 6.1 (2015): 1-12. PMID 26028404
[25] Kojima, Koji, and Charles A. Vacanti. "Tissue engineering in the trachea." The Anatomical Record 297.1 (2014): 44-50. PMID 24293389
[26] "NYU Voice Center - Tracheal Tumor." Med.nyu. NYU, 2005. Web. 13 Apr. 2012. <http://www.med.nyu.edu/voicecenter/resources/photo/tracheal_tumor.html>.
