User:Victor Tapia: Difference between revisions
Victor Tapia (talk | contribs) |
Victor Tapia (talk | contribs) |
||
| (185 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
< | ==Contact Information== | ||
[[Image:Tapia_bewerbung.jpg|200px|right]] | |||
<div style="padding: 10px; color: #000; background-color: #CEF2E0; width: 500px"> | |||
Dr. rer. medic. <br> | |||
[[Image: | Víctor E . Tapia Mancilla <br> | ||
Email: ve.tapia.m@gmail.com <br> | |||
<br> | |||
Inst. f. Med. Immunol., <br> | |||
Charité - Universitätsmedizin Berlin, CCM <br> | |||
Hessische Str. 3-4, D-10115 Berlin <br> | |||
+49-30-450 524285 <br><br> | |||
Former institutions:<br> | |||
{{hide|<br> | |||
[[Image:Molrec placeholder.png|167px|right]] | |||
Charité - Universitätsmedizin Berlin<br> | |||
[http://immunologie.charite.de/ Institut für medizinische Immunologie<br>] | |||
AG Molekulare Bibliotheken<br> | |||
Dr. Rudolf Volkmer<br> | |||
Hessische Str. 3-4, 10115 Berlin<br> | |||
<br> | |||
Tel.: +49-30-450 524267<br> | |||
oder +49-30-450 524317<br> | |||
Fax: +49-30-450 524962<br> | |||
E-mail: rve@charite.de | |||
<br><br>- - - - - - - - - - - - - - - - - - - - - - - - <br><br> | |||
<br> | |||
[[Image:Molrec placeholder.png|167px|right]] | |||
UMR7156 CNRS-Université de Strasbourg<br> | |||
Equipe Cytosquelette d'actine et Trafic intracellulaire<br> | |||
Département de Biologie moléculaire et cellulaire<br> | |||
Barbara Winsor, PhD<br> | |||
[http://www.endocytosis.org/F-BAR_proteins/JMConference/barbara_winsor.html († 29.09.2011)]<br> | |||
<br><br>- - - - - - - - - - - - - - - - - - - - - - - - <br><br> | |||
<br> | |||
[[Image:Molrec placeholder.png|167px|right]] | |||
Humboldt Universität, Berlin<br> | |||
Institute for Theoretical Biology<br> | |||
Systems Immunology<br> | |||
Michal Or-Guil<br> | |||
<br><br>- - - - - - - - - - - - - - - - - - - - - - - - <br><br> | |||
}} | |||
Social networks<br> | |||
{{hide|<br> | |||
<html> | |||
<a href="http://www.mendeley.com/profiles/ve-tapia"><img border="0" src="http://www.mendeley.com/embed/icon/2/red/small" alt="Bibliography manager"/></a> | |||
</html><br> | |||
[http://victortapia.brandyourself.com jet another profile]<br> | |||
[http://de.linkedin.com/pub/victor-tapia/29/285/a43 jet another other profile]<br> | |||
[https://www.researchgate.net/profile/Victor_Tapia3]<br> | |||
<br>- - - - - - - - - - - - - - - - - - - - - - - - <br><br> | |||
}} | |||
last actualization: 01.07.2014 | |||
</div> | |||
* | ==Research interests== | ||
* | <!-- Feel free to add brief descriptions to your research interests as well --> | ||
* | <div style="padding: 10px; color: #000; background-color: #CEF2E0; width: 500px"> | ||
* | * [http://openwetware.org/wiki/Molecular_Recognition_Laboratorium#Structural_Modularity__in_Protein-Protein_Recognition Modularity of Protein Structure and Cellular Signal Transduction]. | ||
* | * Protein Folding and Neurodegenerative Deseases. | ||
* Natural Autoimmunology and Diagnostic Assays | |||
<br><br> | |||
'''Scientific Projects with Principal Responsability''' <br> | |||
2010 <br> | |||
* Complete SH3 Binding Profiles across Bacterial Strains for the Analysis of Protein Interaction Network Evolution. <br> | |||
* BAG3-WW Binding Profile to Elucidate the Protein's Function. <br> | |||
2008 <br> | |||
* PQBP1-WW Binding Profile to Elucidate the Molecular Mechanisms of the IGH-Syndrome. <br> | |||
2007 <br> | |||
* Proteosomal Degradation of Immobilized Peptides. <br> | |||
2006 <br> | |||
* Quality Control of Production and Application Procedures in the Peptide-Microarray Technique. <br> | |||
* Characterization of Sequence Elements of A-beta Self Recognition. <br> | |||
2004 <br> | |||
* Diagnosis of Immunization States with Patterns of Natural Autoimmune Reactivities. <br> | |||
</div> | |||
<br><br> | |||
==Leseproben== | |||
[[Image:Pqbp1 arch.png|400px|right]] | |||
<div style="padding: 10px; color: #000; background-color: #CEF2E0; width: 500px"> | |||
===GOLABI-ITO-HALL SYNDROM (GIHS)=== | |||
Das Gen fürs polyglutamine-tract binding Protein 1 (PQBP1), welches auf dem X-Chromosom lokalisiert ist, kodiert für ein 38 kDa Zellkernprotein das überwiegend im zentralen Nervensystem exprimiert wird. In der Literatur wird die Rolle des PQBP1 Proteins in der Entwicklung von auf Polyglutaminexpansion beruhenden Krankheiten wie z. B der spinozerebellären Ataxie Typ 1 beschrieben (Okazawa et al., 2002). Weiterhin sind Mutationen im PQBP1 Gen in mehreren X-Chromosom gebundenen Retardierungen (XLMR), wie dem Renpenning, Sutherland-Haan, Hamel, Porteous und Golabi-Ito-Hall Syndrom (GIHS), nachgewiesen worden (Ropers and Hamel, 2005). Dieser XLMR Syndrome assoziieren mit unterschiedlichen Mutationen des PQBP1 Gens, und dennoch teilen sie ähnliche klinische Merkmale. | |||
Die Y65C PQBP1 Mutation, die mit dem GIH Syndrom assoziiert (Lubs et al., 2006), ist einzigartig unter den bisher gemeldeten PQBP1 Mutationen. Die Läsion beeinflusst nicht die Länge des mutierten Proteins sondern betrifft eine punktuelle Position innerhalb des WW Erkennungsmodules. Mutationen, die in Erkennungsmodulen oder in ihren zugehörigen Liganden gefunden werden, beeinflussen die involvierten Proteinkomplexe und die damit verbundenen Signalwege und können zu Krankheiten führen. | |||
QUELLE: the image to right was made in Inkscape | |||
</div><br> | |||
[[Image:CASA.png|400px|right]] | |||
<div style="padding: 10px; color: #000; background-color: #CEF2E0; width: 500px"> | |||
===Chaperon-assistierte selektive Autophagie (CASA)=== | |||
CASA ist entscheidend um die Muskelaktivität unter mechanischer Spannung in Fliegen, Mäusen und Menschen (Arndt et al., 2010; Homma et al., 2006; Selcen et al., 2009) zu erhalten (Abbildung 2). Eine Beeinträchtigung der CASA verursacht schwere Muskeldystrophien und eine dilatierte Kardiomyopathie, und wurde mit der Gliedergürteldystrophie (Arimura et al., 2011; Homma et al., 2006; Sarparanta et al., 2012) in Verbindung gebracht. | |||
In Muskelzellen spielt BAG3 eine Schlüsselrolle wo es hoch exprimiert und überwiegend an der Z-Scheibe lokalisiert ist. Die Z-Scheibe ist ein Proteinkomplex und dient der Aktin-Verankerung in der quer-gestreiften Muskulatur. Für die Verankerung ist das Z-Scheiben-Protein Filamin wichtig, da es als verbindende Brücke zwischen Aktin und Integrinen in der sakroplasmatischen Membran wirkt. In der kontrahierenden Muskulatur wird das Filamin mechanisch stark beansprucht und häufig entfaltet und unterliegt dann einem Abbau durch CASA. Dieser Abbau wird duch BAG3 eingeleitet. | |||
Nach Kontraktion-induzierter Entfaltung, wird Filamin von einem Chaperon-Komplex anerkannt. Der Komplex enthält Hsc70 und HspB8 (auch als Hsp22 bekannt), die physikalisch vom CASA-induzierende Ko-Chaperon BAG3 zusammengehalten werden. Filamin wird aus der Z-Scheibe freigegeben und durch die Hsc70-assoziierte Ubiquitinligase CHIP ubiquitiniert. Es wird dann von dem autophagischen Ubiquitin Adaptor Protein p62 dem lysosomalen Verdau zugeführt, indem p62 mit Phagophorenmembranen interagiert. Der autophagische Abbau von Filamin ist eine Voraussetzung für die ordnungsmäßige Funktion von Z-Scheiben in kontrahierender, quergestreifter Muskulatur (Arndt et al., 2010). | |||
QUELLE: Text and image to the right were adapted from the following sources:<br> | |||
* Ulbricht, A., Eppler, F.J., Tapia, V.E., Van der Ven, P.F.M., Hampe, N., Hersch, N., Vakeel, P., Stadel, D., Haas, A., Saftig, P., Behrends, C., Fürst, D.O., Volkmer, R., Hoffmann, B., Kolanus, W., Höhfeld, J., 2013. Cellular Mechanotransduction Relies on Tension-Induced and Chaperone-Assisted Autophagy. Accepted by Current Biology <br> | |||
* Rogon & Höhfeld, 2010. Proteostase – Chaperone als Begleiter von der Wiege bis zum Grabe. Biospektrum 4/2010; 413 | |||
</div><br> | |||
[[Image:Penelope fig1.png|400px|right]] | |||
<div style="padding: 10px; color: #000; background-color: #CEF2E0; width: 500px"> | |||
===INTERAKTIONSNETZWERKE=== | |||
Zur Jahrhundertwende wurden einige in vivo/ex vivo Strategien entwickelt, um proteomische Interaktionsnetzwerke zu charakterisieren (Gavin et al., 2002; Ho et al., 2002; Ito et al., 2001; Uetz et al., 2000). Es zeigte sich jedoch sehr schnell, dass die berichteten Interaktome aus Saccharomyces cerevisiae nur eine mäßige Überlappung zeigen. Diese Erkenntnis fordert zwingend jegliche Interaktomstudien durch orthogonale Methoden zu validieren (Von Mering et al., 2002). | |||
Eine alternative in vitro Strategie basiert auf einer Hochdurchsatz-Protein-Präparierung im Arrayformat (Kung et al., 2009; Phizicky et al., 2003; Ptacek et al., 2005; Zhu et al., 2001). Obwohl inzwischen einige experimentelle Studien publiziert wurden, ist noch nicht klar, welcher Prozentsatz eines eukaryotischen Proteoms in gefalteter Form hergestellt und funktionsfähig auf einen festen Träger immobilisiert werden kann. | |||
Im Gegensatz zur bestehenden Unsicherheit bei Proteinarrays können hochdichte Peptidarrays effizient durch eine Spot Synthese präpariert werden (Frank, 2002; Hilpert et al., 2007; Winkler et al., 2011). Der Informationsverlust durch Verzicht auf den Proteinkontext wird durch die Tatsache relativiert, dass erkannte kurze lineäre motiven in nativen unstrukturierten Regionen der Proteinarchitektur exponiert werden (Dinkel et al., 2011; Fuxreiter et al., 2007). | |||
Der Vorteil des Peptidarray-Formats erschließt sich für jene Protein-Protein-Wechselwirkungen, in denen einer der Bindungspartner die Komplexbildung durch Andocken an einen kurzen linearen Sequenzabschnitt seines Partnerproteins auslöst . In der Tat wird eine beträchtliche Menge von Protein-Protein-Interaktionen durch Familien von Erkennungsdomänen vermittelt (WW, SH3, SH2, PDZ usw.), die kurze lineare Peptide in ihrer Bindungstaschen aufnehmen (Hunter and Pawson, 2012; Pawson and Linding, 2008, 2005; Pawson and Nash, 2000). | |||
Eine vergleichende Analyse der Evolution von SH3-Netzwerke wurde zusammen mit Kooperationspartnern, wie in 3 skizziert, durchgeführt (Verschueren et al., n.d.). | |||
QUELLE: The image on the right was adapted from | |||
* Tonikian, R., Xin, X., Toret, C.P., Gfeller, D., Landgraf, C., Panni, S., Paoluzi, S., Castagnoli, L., Currell, B., Seshagiri, S., Yu, H., Winsor, B., Vidal, M., Gerstein, M.B., Bader, G.D., Volkmer, R., Cesareni, G., Drubin, D.G., Kim, P.M., Sidhu, S.S., Boone, C., 2009. Bayesian Modeling of the Yeast SH3 Domain Interactome Predicts Spatiotemporal Dynamics of Endocytosis Proteins. PLoS Biol 7, e1000218. | |||
</div><br> | |||
==TALKING TO MY SELF (?)== | |||
[[Image:Hitchhicking-on-rnap2.png|400px|right]] | |||
<div style="padding: 10px; color: #000; background-color: #CEF2E0; width: 500px"> | |||
===ON TAPIA ET AL. 2010=== | |||
On a collaborative project concerning the role of PQBP1 in X-linked intellectual disability (X-LID), the inherent specificity of the wild-type and the Y65C-mutant PQBP1 WW were directly compared (Tapia et al., 2010). The collection of probes used to profile the linear motif recognition specificity contained a core repertoire of PPXY motifs and an additional tailor-made collection of non-PPXY potential ligands based on the literature. An independent comparison of the specificity of both WW versions was also carried out. Therein, the repertoire of probes represented a complete and redundant permutation of poS postitions in a sequence consensus of the CTD repeats in the human RNAPII (YS2PTS5PS-YS2PTS5PS). | |||
<br><br> | |||
Earlier reported interactions with phosphorylated CTD from RNAPII (Okazawa et al., 2002) and with SIPP1, a splicing factor interacting with Ser/Thr phosphatase-1 (PP1) protein (Llorian et al., 2005), were confirmed. Moreover, a comparison of wt and Y65C-mutant PQBP1 WW specificity profiles shows that the mutation, known to be associated with GIHS (Lubs et al., 2006), compromises the recognition of SIPP1. This effect was accordingly observed in cell extracts from HEK98 and lymphoblasts isolated from a GIHS patient (Figure 5A and B). Biophysical analysis of the mutation effect additionally showed a compromised thermal stability of the WW structure and reduced binding to SIPP1. | |||
<br><br> | |||
The consequences of the compromised PQBP1/SIPP1 complex was a significant reduction of pre-mRNA splicing, as shown in lymphoblasts derived from a GIHS patient. The decreased splicing efficiency was similar to that seen after small interfering RNA-mediated knockdown of PQBP1, indicating that PQBP1-Y65C is inactive in intact cells (Figure 5C-G). | |||
Moreover, Tapia et al. (2010) shows that the known WW-mediation of binding to RNAPII (Okazawa et al., 2002) depends on hyperphosphorylation of RNAPII's CTD. In the cell this is done by the pTEFb kinase complex, which thus imprints a post-translational marker for elongating RNAPII, known to recruit splicing factors (Batsché et al., 2006; Phatnani and Greenleaf, 2006). The orchestrating role of RNAPII in the cross-talk between transcription and splicing is well described (Bird et al., 2004; David and Manley, 2011; Neugebauer, 2002). | |||
<br><br> | |||
These findings provide additional empirical support for a role of PQBP1 in pre-mRNA splicing. Alternative splicing is particularly important in the brain, and a switch in alternative splicing patterns of primary transcripts encoding neuron-specific proteins is known to accompany neuronal differentiation (Fairbrother and Lipscombe, 2008; Lipscombe et al., 2013). Changes in alternative splice choices could, therefore, represent an important factor in the etiology of GIHS. More details in the involvement of PQBP1 and alternative splicing in neurodegeneration could be achieved upon identification of primary transcripts targeted by PQBP1-assisted alternative splicing (Wang et al., 2013). | |||
<br><br> | |||
The diverse lesions in PQBP1 may lead to similar intelectual and morphological symptomes. Besides the Y65C point-mutation, all other X-LID associated PQBP1 mutation produce truncated proteins, which lack a C-terminal intrinsically unstructured domain known to bind the spliceosome assembling factor U5-15kDa. Such facts suggest that the WW domain of PQBP1 is sufficient to cause X-LID but not exclusively necessary as causative agent. It is posible that PQBP1 is firstly recruited by elongating RNAPII, then co-localizes to the assembling spliceosome through its C-terminal domain and, driven either by affinity or effective specificity, dynamic WW-mediated recognition may switch to SIPP1 binding to help activate the catalytic steps of pre-mRNA splicing. Alternatively, the role of RNAPII recognition may be secondary to the recognition of SIPP1, which has been shown to be shuttled to the nucleous independently from its own predicted nuclear localization signals, most possibly by PQBP1 (Llorian et al., 2005). Thus, under this model, PQBP1 functions as a scaffold between spliceosome assembly (C-terminal domain-mediated) and catalytic activity (WW-domain mediated). | |||
<br><br> | |||
This idea is apparently in paradox with the fact, that studies of PQBP1 involvement in intellectual disabilities using animal models show that Mus musculus and Drosophila melanogaster with knocked-down PQBP1 may be rescued from developing symptoms analogous to X-LID syndromes by applying HDAC inhibiting drugs (Ito et al., 2009; Tamura et al., 2010). Recent hypotheses of a cross-talk between chromatin remodelling and alternative splicing (Allemand et al., 2008) may shed some light on these findings. In the later citation, the authors are “tempted to speculate that the splicing machinery relies on chromatin regulators which are able to read the ‘histone code’ to locate and access pre-mRNAs awaiting splicing”. If they are given truth, the apparent paradox would turn to a further evidence. | |||
<br><br> | |||
Less conflictive are reports of PQBP1 transcription regulating activity through recognition of poly-Q expanded tracts in the transcription factors Brn-2 (Waragai et al., 1999) and ataxin-1 (Okazawa et al., 2002; Okuda et al., 2003). Indeed, SFs and TFs show common elements in their interaction networks and are oft erroneously categorized (Brès et al., 2005; Expert-Bezançon et al., 2002; Hastings et al., 2007). Poly-Q expanded ataxin-1 was shown to increases the affinity of the PQBP1-WW for the phosphorylated and active form of the RNAPII-CTD, leading to its dephosphorylation (Okazawa et al., 2002). Desphosphorylation on S2 and diffident reappearance of S5 phosphorylation on the CTD of eleongating RNAPII is known to slow down elongation rates and favor the recognition of weaker consensus sequences for splicing factor (de la Mata et al., 2003). Such situation favors the usage of alternative splicing patterns and the outcome of splicing variated gene products. | |||
<br><br> | |||
Thus, the empirical support for the involvement of PQBP1 in X-LID via alternative RNA splicing is found to fit and complement diverse empirical findings and theoretical postulates independently reported by different research laboratories. | |||
</div><br> | |||
==Education== | ==Education== | ||
< | <div style="padding: 10px; color: #000; background-color: #CEF2E0; width: 500px"> | ||
May 2010 – April 2011 | <br> | |||
Fellow scientific associate in “Penelope” (EU Marie Curie Research Training Network, MRTN-CT-2006- 0036076) coordinated by Dr. Luis Serrano, Centre for Genomic Regulation, Barcelona | |||
July 2008 – April 2010 | <br> | |||
Fellow scientific associate in “Structure and Function of Membrane Receptors” (Collaborative Research Centre, SFB 449) | |||
coordinated by Prof. Dr. Volker Haucke, Freie Universität, Berlin | |||
Sept. 2003 – June 2004 | <br> | |||
Diploma thesis in the Systems Immunology Lab | |||
led by Dr. M. Or-Guil at the Institute for theoretical Biology of the Humboldt Universität in collaboration with the Charité Universitätsklinikum, Berlin | |||
Oct. 1998 – June 2004 | <br> | |||
Study of Biology with a Biochemistry major at the Humboldt Universität, Berlin | |||
May 1996 – Sept. 1998 | <br> | |||
German as Foreign Language, German Literature and Philosophy, Epistemology, and Sociobiology as guest student at the RWTH, Aachen. | |||
March 1979 – Dec. 1991 | <br> | |||
< | School in Viña del Mar, Chile and Philadelphia, PA, USA. | ||
</div> | |||
<br><br> | |||
==Publications== | ==Publications== | ||
< | [[Image:victor_tapia.jpg|200px|right]] | ||
<div style="padding: 10px; color: #000; background-color: #CEF2E0; width: 500px"> | |||
* 10 - M Spiess, E Verschueren, VE TAPIA, PM Kim, A Norgaard, C Landgraf, R Volkmer, F Hochstenbach, B Distel, B Winsor, L Serrano (2012). “EVOLUTION OF THE SH3 DOMAIN INTERACTOME ACROSS YEAST SPECIES” | |||
*:''[Manuscript in preparation].'' | |||
* 9 - Anna Ulbricht, Felix J. Eppler, VE TAPIA, Peter F.M. van der Ven, Padmanabhan Vakeel, Daniela Stadel, Albert Haas, Bernd Hoffmann, Paul Saftig, Christian Behrends, Dieter O. Fürst, Rudolf Volkmer, Waldemar Kolanus & Jörg Höhfeld. (2012) “CELLULAR MECHANOTRANSDUCTION RELIES ON TENSION-INDUCED AND CHAPERONE-ASSISTED AUTOPHAGY“ | |||
// This work was carried out to adapt "building block" approaches for the generation of phosphopeptides through resine-supported solid-phase peptide synthesis to the spot-technology, which relies on cellulose as support. It compares different coupling strategies for the incorporation of protected | *:''[Manuscript submitted on June 29 2012]'' | ||
* 8 - Volkmer, R & V Tapia. (2011) “EXPLORING PROTEIN-PROTEIN INTERACTIONS WITH SYNTHETIC PEPTIDE ARRAYS”. Mini-Reviews in Organic Chemistry 8(2): 164. | |||
*:''[http://www.ingentaconnect.com/content/ben/mroc/2011/00000008/00000002/art00009 View Abstract from MROC]'' | |||
* 7 - Volkmer, R, I Kretzschmar & VE TAPIA. (2011) “Mapping receptor-ligand in-teractions with synthetic peptide arrays: Exploring the structure and function of membrane receptors”. European Journal of Cell Biology 91(4): 349. | |||
*:''[http://dx.doi.org/10.1016/j.ejcb.2011.03.004 View Abstract from EJCB]'' | |||
< | * 6 - Tapia VE, Nicolaescu E, McDonald CB, Musi V, Oka T, Inayoshi Y, Satteson AC, Mazack V, Humbert J, Gaffney CJ, Beullens M, Schwartz CE, Landgraf C, Volkmer R, Pastore A, Farooq A, Bollen M, Sudol M. (2010) "Y65C MISSENSE MUTATION IN THE WW DOMAIN OF THE GOLABI-ITO-HALL SYNDROME PROTEIN PQBP1 AFFECTS ITS BINDING ACTIVITY AND DEREGULATES PRE-mRNA SPLICING." J Biol Chem.;285(25):19391-401. Epub 2010 Apr 21. | ||
< | *:''This work explores the effect of a point mutation leading to X-LMR at the reductionist level of domain-peptide interactions, inferes potential affected functions, and tests inferences on pre-mRNA splicing. | ||
*:''[http://www.jbc.org/content/285/25/19391.full.pdf+html/ Download from JBC]'' | |||
* 5 - Mahrenholz CC, Tapia V, Stigler RD, Volkmer R. (2010) "A STUDY TO ASSESS THE CROSS-REACTIVITY OF CELLULOSE MEMBRANE-BOUND PEPTIDES WITH DETECTION SYSTEMS: AN ANALYSIS AT THE AMINO ACID LEVEL." J Pept Sci.;16(6):297-302. | |||
*:''ever wanted to see if your favorite detection system reacts with cellulose-bound peptides? Well, chances are given that it is one of the three covered here: TAMRA-dye, luminol turn-over, and FITC'' | |||
* 4 - Tapia VE & Volkmer R (2009) "EXPLORING AND PROFILING PROTEIN FUNCTION WITH PEPTIDE ARRAYS" in: Methods in Molecular Biology, Vol 570 (Eds. M Cretich & M Chiari). Humana Press. - ISBN: 978-1-60327-393-0. | |||
*:''This work reviews the peptide array technology focusing on technological advancements, oriented immobilization of peptide probes, and some interesting applications. Going along the philosophy of the monography series, we give some general recomendations on methods and instruments. See the following newsletter comments by Tony Fong: Q&A: Raising the Profile of Peptide Arrays for Studying Protein Function, ProteoMonitor, August 13, 2009.'' | |||
*:''[http://www.ncbi.nlm.nih.gov/pubmed/19649587/ View abstract at PubMed]'' | |||
*:''[http://www.genomeweb.com/proteomics/qa-raising-profile-peptide-arrays-studying-protein-function Read the newsletter by Tony Fong]'' | |||
* 3 - Tapia VE, Ay B, Triebus J, Wolter E, Boisguerin P, Volkmer R. EVALUATING THE COUPLING EFFICIENCY OF PHOSPHORYLATED AMINO ACIDS FOR SPOT SYNTHESIS. J Pept Sci. 2008 Dez ;14(12):1309-1314. - PMID: 18816512. | |||
*:''This work was carried out to adapt "building block" approaches for the generation of phosphopeptides through resine-supported solid-phase peptide synthesis to the spot-technology, which relies on cellulose as support. It compares different coupling strategies for the incorporation of protected phoshoaminoacids into determined positions of synthetic peptides. We find that the use of EEDQ as coupling activator is the most efficient strategy in our comparison.'' | |||
*:''[http://www.ncbi.nlm.nih.gov/pubmed/18816512 view abstract at PubMed]'' | |||
* 2 - Tapia VE, Bongartz J, Schutkowski M, Bruni N, Weiser A, Ay B, Volkmer R, Or-Guil M. AFFINITY PROFILING USING THE PEPTIDE MICROARRAY TECHNOLOGY: A CASE STUDY. Anal Biochem. 2007 Apr 1;363(1):108-118. - PMID: 17288979. | |||
*:''This work analyses the predictive potential of microarray-based binding assays. Fluorescent SI-measurements were used to fit a mass-action derived model estimating the observed affinity (equil. diss. const.).'' | |||
*:''[http://www.ncbi.nlm.nih.gov/pubmed/17288979/ View abstract at PubMed]'' | |||
* 1 - Weiser AA, Or-Guil M, Tapia VE, Leichsenring A, Schuchhardt J, Frommel C, Volkmer-Engert R. SPOT SYNTHESIS: RELIABILITY OF ARRAY-BASED MEASUREMENT OF PEPTIDE BINDING AFFINITY. Anal Biochem. 2005 Juli 15;342(2):300-311. - PMID: 15950918. | |||
*:''This work analyses the quantitative potential of measurements derived from peptide macroarray-based immunobinding assays. Chemoluminiscent signal intensity data was confronted with independent "golden stardard" measurements (surface plasmon resonance-based binding assays on a BIAcoreX maschine).'' | |||
</div><br> | |||
==Useful links== | ==Useful links== | ||
<div style="padding: 10px; color: #ffffff; background-color: #000; width: 600px"> | |||
*[[OpenWetWare:Welcome|Introductory tutorial]] | *[[OpenWetWare:Welcome|Introductory tutorial]] | ||
*[[Help|OpenWetWare help pages]] | *[[Help|OpenWetWare help pages]] | ||
*[http://openwetware.org/wiki/Molecular_Recognition_Laboratorium Molecular Recognition Lab] | |||
*[http://immunologie.charite.de/arbeitsgruppen/ag-volkmer AG-Volkmer official site] | |||
*[http://meta.wikimedia.org/wiki/Help:Special_page wiki editing] | |||
</div> | |||
Revision as of 01:05, 27 July 2014
Contact Information

Dr. rer. medic.
Víctor E . Tapia Mancilla
Email: ve.tapia.m@gmail.com
Inst. f. Med. Immunol.,
Charité - Universitätsmedizin Berlin, CCM
Hessische Str. 3-4, D-10115 Berlin
+49-30-450 524285
Former institutions:

Charité - Universitätsmedizin Berlin
Institut für medizinische Immunologie
AG Molekulare Bibliotheken
Dr. Rudolf Volkmer
Hessische Str. 3-4, 10115 Berlin
Tel.: +49-30-450 524267
oder +49-30-450 524317
Fax: +49-30-450 524962
E-mail: rve@charite.de
- - - - - - - - - - - - - - - - - - - - - - - -

UMR7156 CNRS-Université de Strasbourg
Equipe Cytosquelette d'actine et Trafic intracellulaire
Département de Biologie moléculaire et cellulaire
Barbara Winsor, PhD
(† 29.09.2011)
- - - - - - - - - - - - - - - - - - - - - - - -

Humboldt Universität, Berlin
Institute for Theoretical Biology
Systems Immunology
Michal Or-Guil
- - - - - - - - - - - - - - - - - - - - - - - -
Social networks
<html>
<a href="http://www.mendeley.com/profiles/ve-tapia"><img border="0" src="http://www.mendeley.com/embed/icon/2/red/small" alt="Bibliography manager"/></a>
</html>
jet another profile
jet another other profile
[1]
- - - - - - - - - - - - - - - - - - - - - - - -
last actualization: 01.07.2014
Research interests
- Modularity of Protein Structure and Cellular Signal Transduction.
- Protein Folding and Neurodegenerative Deseases.
- Natural Autoimmunology and Diagnostic Assays
Scientific Projects with Principal Responsability
2010
- Complete SH3 Binding Profiles across Bacterial Strains for the Analysis of Protein Interaction Network Evolution.
- BAG3-WW Binding Profile to Elucidate the Protein's Function.
2008
- PQBP1-WW Binding Profile to Elucidate the Molecular Mechanisms of the IGH-Syndrome.
2007
- Proteosomal Degradation of Immobilized Peptides.
2006
- Quality Control of Production and Application Procedures in the Peptide-Microarray Technique.
- Characterization of Sequence Elements of A-beta Self Recognition.
2004
- Diagnosis of Immunization States with Patterns of Natural Autoimmune Reactivities.
Leseproben
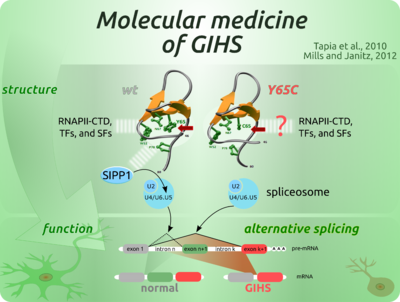
GOLABI-ITO-HALL SYNDROM (GIHS)
Das Gen fürs polyglutamine-tract binding Protein 1 (PQBP1), welches auf dem X-Chromosom lokalisiert ist, kodiert für ein 38 kDa Zellkernprotein das überwiegend im zentralen Nervensystem exprimiert wird. In der Literatur wird die Rolle des PQBP1 Proteins in der Entwicklung von auf Polyglutaminexpansion beruhenden Krankheiten wie z. B der spinozerebellären Ataxie Typ 1 beschrieben (Okazawa et al., 2002). Weiterhin sind Mutationen im PQBP1 Gen in mehreren X-Chromosom gebundenen Retardierungen (XLMR), wie dem Renpenning, Sutherland-Haan, Hamel, Porteous und Golabi-Ito-Hall Syndrom (GIHS), nachgewiesen worden (Ropers and Hamel, 2005). Dieser XLMR Syndrome assoziieren mit unterschiedlichen Mutationen des PQBP1 Gens, und dennoch teilen sie ähnliche klinische Merkmale. Die Y65C PQBP1 Mutation, die mit dem GIH Syndrom assoziiert (Lubs et al., 2006), ist einzigartig unter den bisher gemeldeten PQBP1 Mutationen. Die Läsion beeinflusst nicht die Länge des mutierten Proteins sondern betrifft eine punktuelle Position innerhalb des WW Erkennungsmodules. Mutationen, die in Erkennungsmodulen oder in ihren zugehörigen Liganden gefunden werden, beeinflussen die involvierten Proteinkomplexe und die damit verbundenen Signalwege und können zu Krankheiten führen.
QUELLE: the image to right was made in Inkscape

Chaperon-assistierte selektive Autophagie (CASA)
CASA ist entscheidend um die Muskelaktivität unter mechanischer Spannung in Fliegen, Mäusen und Menschen (Arndt et al., 2010; Homma et al., 2006; Selcen et al., 2009) zu erhalten (Abbildung 2). Eine Beeinträchtigung der CASA verursacht schwere Muskeldystrophien und eine dilatierte Kardiomyopathie, und wurde mit der Gliedergürteldystrophie (Arimura et al., 2011; Homma et al., 2006; Sarparanta et al., 2012) in Verbindung gebracht. In Muskelzellen spielt BAG3 eine Schlüsselrolle wo es hoch exprimiert und überwiegend an der Z-Scheibe lokalisiert ist. Die Z-Scheibe ist ein Proteinkomplex und dient der Aktin-Verankerung in der quer-gestreiften Muskulatur. Für die Verankerung ist das Z-Scheiben-Protein Filamin wichtig, da es als verbindende Brücke zwischen Aktin und Integrinen in der sakroplasmatischen Membran wirkt. In der kontrahierenden Muskulatur wird das Filamin mechanisch stark beansprucht und häufig entfaltet und unterliegt dann einem Abbau durch CASA. Dieser Abbau wird duch BAG3 eingeleitet. Nach Kontraktion-induzierter Entfaltung, wird Filamin von einem Chaperon-Komplex anerkannt. Der Komplex enthält Hsc70 und HspB8 (auch als Hsp22 bekannt), die physikalisch vom CASA-induzierende Ko-Chaperon BAG3 zusammengehalten werden. Filamin wird aus der Z-Scheibe freigegeben und durch die Hsc70-assoziierte Ubiquitinligase CHIP ubiquitiniert. Es wird dann von dem autophagischen Ubiquitin Adaptor Protein p62 dem lysosomalen Verdau zugeführt, indem p62 mit Phagophorenmembranen interagiert. Der autophagische Abbau von Filamin ist eine Voraussetzung für die ordnungsmäßige Funktion von Z-Scheiben in kontrahierender, quergestreifter Muskulatur (Arndt et al., 2010).
QUELLE: Text and image to the right were adapted from the following sources:
- Ulbricht, A., Eppler, F.J., Tapia, V.E., Van der Ven, P.F.M., Hampe, N., Hersch, N., Vakeel, P., Stadel, D., Haas, A., Saftig, P., Behrends, C., Fürst, D.O., Volkmer, R., Hoffmann, B., Kolanus, W., Höhfeld, J., 2013. Cellular Mechanotransduction Relies on Tension-Induced and Chaperone-Assisted Autophagy. Accepted by Current Biology
- Rogon & Höhfeld, 2010. Proteostase – Chaperone als Begleiter von der Wiege bis zum Grabe. Biospektrum 4/2010; 413

INTERAKTIONSNETZWERKE
Zur Jahrhundertwende wurden einige in vivo/ex vivo Strategien entwickelt, um proteomische Interaktionsnetzwerke zu charakterisieren (Gavin et al., 2002; Ho et al., 2002; Ito et al., 2001; Uetz et al., 2000). Es zeigte sich jedoch sehr schnell, dass die berichteten Interaktome aus Saccharomyces cerevisiae nur eine mäßige Überlappung zeigen. Diese Erkenntnis fordert zwingend jegliche Interaktomstudien durch orthogonale Methoden zu validieren (Von Mering et al., 2002). Eine alternative in vitro Strategie basiert auf einer Hochdurchsatz-Protein-Präparierung im Arrayformat (Kung et al., 2009; Phizicky et al., 2003; Ptacek et al., 2005; Zhu et al., 2001). Obwohl inzwischen einige experimentelle Studien publiziert wurden, ist noch nicht klar, welcher Prozentsatz eines eukaryotischen Proteoms in gefalteter Form hergestellt und funktionsfähig auf einen festen Träger immobilisiert werden kann. Im Gegensatz zur bestehenden Unsicherheit bei Proteinarrays können hochdichte Peptidarrays effizient durch eine Spot Synthese präpariert werden (Frank, 2002; Hilpert et al., 2007; Winkler et al., 2011). Der Informationsverlust durch Verzicht auf den Proteinkontext wird durch die Tatsache relativiert, dass erkannte kurze lineäre motiven in nativen unstrukturierten Regionen der Proteinarchitektur exponiert werden (Dinkel et al., 2011; Fuxreiter et al., 2007). Der Vorteil des Peptidarray-Formats erschließt sich für jene Protein-Protein-Wechselwirkungen, in denen einer der Bindungspartner die Komplexbildung durch Andocken an einen kurzen linearen Sequenzabschnitt seines Partnerproteins auslöst . In der Tat wird eine beträchtliche Menge von Protein-Protein-Interaktionen durch Familien von Erkennungsdomänen vermittelt (WW, SH3, SH2, PDZ usw.), die kurze lineare Peptide in ihrer Bindungstaschen aufnehmen (Hunter and Pawson, 2012; Pawson and Linding, 2008, 2005; Pawson and Nash, 2000). Eine vergleichende Analyse der Evolution von SH3-Netzwerke wurde zusammen mit Kooperationspartnern, wie in 3 skizziert, durchgeführt (Verschueren et al., n.d.).
QUELLE: The image on the right was adapted from
- Tonikian, R., Xin, X., Toret, C.P., Gfeller, D., Landgraf, C., Panni, S., Paoluzi, S., Castagnoli, L., Currell, B., Seshagiri, S., Yu, H., Winsor, B., Vidal, M., Gerstein, M.B., Bader, G.D., Volkmer, R., Cesareni, G., Drubin, D.G., Kim, P.M., Sidhu, S.S., Boone, C., 2009. Bayesian Modeling of the Yeast SH3 Domain Interactome Predicts Spatiotemporal Dynamics of Endocytosis Proteins. PLoS Biol 7, e1000218.
TALKING TO MY SELF (?)
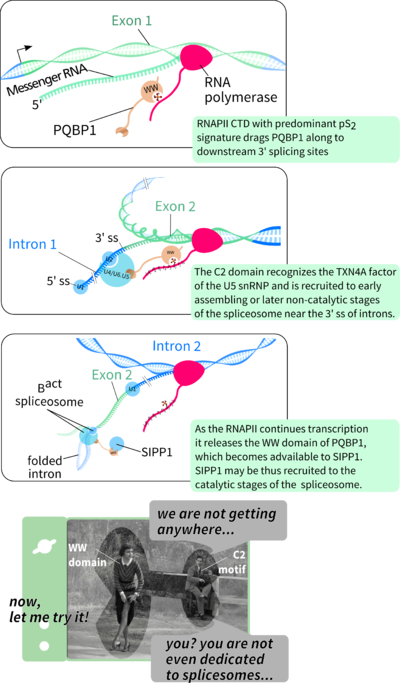
ON TAPIA ET AL. 2010
On a collaborative project concerning the role of PQBP1 in X-linked intellectual disability (X-LID), the inherent specificity of the wild-type and the Y65C-mutant PQBP1 WW were directly compared (Tapia et al., 2010). The collection of probes used to profile the linear motif recognition specificity contained a core repertoire of PPXY motifs and an additional tailor-made collection of non-PPXY potential ligands based on the literature. An independent comparison of the specificity of both WW versions was also carried out. Therein, the repertoire of probes represented a complete and redundant permutation of poS postitions in a sequence consensus of the CTD repeats in the human RNAPII (YS2PTS5PS-YS2PTS5PS).
Earlier reported interactions with phosphorylated CTD from RNAPII (Okazawa et al., 2002) and with SIPP1, a splicing factor interacting with Ser/Thr phosphatase-1 (PP1) protein (Llorian et al., 2005), were confirmed. Moreover, a comparison of wt and Y65C-mutant PQBP1 WW specificity profiles shows that the mutation, known to be associated with GIHS (Lubs et al., 2006), compromises the recognition of SIPP1. This effect was accordingly observed in cell extracts from HEK98 and lymphoblasts isolated from a GIHS patient (Figure 5A and B). Biophysical analysis of the mutation effect additionally showed a compromised thermal stability of the WW structure and reduced binding to SIPP1.
The consequences of the compromised PQBP1/SIPP1 complex was a significant reduction of pre-mRNA splicing, as shown in lymphoblasts derived from a GIHS patient. The decreased splicing efficiency was similar to that seen after small interfering RNA-mediated knockdown of PQBP1, indicating that PQBP1-Y65C is inactive in intact cells (Figure 5C-G).
Moreover, Tapia et al. (2010) shows that the known WW-mediation of binding to RNAPII (Okazawa et al., 2002) depends on hyperphosphorylation of RNAPII's CTD. In the cell this is done by the pTEFb kinase complex, which thus imprints a post-translational marker for elongating RNAPII, known to recruit splicing factors (Batsché et al., 2006; Phatnani and Greenleaf, 2006). The orchestrating role of RNAPII in the cross-talk between transcription and splicing is well described (Bird et al., 2004; David and Manley, 2011; Neugebauer, 2002).
These findings provide additional empirical support for a role of PQBP1 in pre-mRNA splicing. Alternative splicing is particularly important in the brain, and a switch in alternative splicing patterns of primary transcripts encoding neuron-specific proteins is known to accompany neuronal differentiation (Fairbrother and Lipscombe, 2008; Lipscombe et al., 2013). Changes in alternative splice choices could, therefore, represent an important factor in the etiology of GIHS. More details in the involvement of PQBP1 and alternative splicing in neurodegeneration could be achieved upon identification of primary transcripts targeted by PQBP1-assisted alternative splicing (Wang et al., 2013).
The diverse lesions in PQBP1 may lead to similar intelectual and morphological symptomes. Besides the Y65C point-mutation, all other X-LID associated PQBP1 mutation produce truncated proteins, which lack a C-terminal intrinsically unstructured domain known to bind the spliceosome assembling factor U5-15kDa. Such facts suggest that the WW domain of PQBP1 is sufficient to cause X-LID but not exclusively necessary as causative agent. It is posible that PQBP1 is firstly recruited by elongating RNAPII, then co-localizes to the assembling spliceosome through its C-terminal domain and, driven either by affinity or effective specificity, dynamic WW-mediated recognition may switch to SIPP1 binding to help activate the catalytic steps of pre-mRNA splicing. Alternatively, the role of RNAPII recognition may be secondary to the recognition of SIPP1, which has been shown to be shuttled to the nucleous independently from its own predicted nuclear localization signals, most possibly by PQBP1 (Llorian et al., 2005). Thus, under this model, PQBP1 functions as a scaffold between spliceosome assembly (C-terminal domain-mediated) and catalytic activity (WW-domain mediated).
This idea is apparently in paradox with the fact, that studies of PQBP1 involvement in intellectual disabilities using animal models show that Mus musculus and Drosophila melanogaster with knocked-down PQBP1 may be rescued from developing symptoms analogous to X-LID syndromes by applying HDAC inhibiting drugs (Ito et al., 2009; Tamura et al., 2010). Recent hypotheses of a cross-talk between chromatin remodelling and alternative splicing (Allemand et al., 2008) may shed some light on these findings. In the later citation, the authors are “tempted to speculate that the splicing machinery relies on chromatin regulators which are able to read the ‘histone code’ to locate and access pre-mRNAs awaiting splicing”. If they are given truth, the apparent paradox would turn to a further evidence.
Less conflictive are reports of PQBP1 transcription regulating activity through recognition of poly-Q expanded tracts in the transcription factors Brn-2 (Waragai et al., 1999) and ataxin-1 (Okazawa et al., 2002; Okuda et al., 2003). Indeed, SFs and TFs show common elements in their interaction networks and are oft erroneously categorized (Brès et al., 2005; Expert-Bezançon et al., 2002; Hastings et al., 2007). Poly-Q expanded ataxin-1 was shown to increases the affinity of the PQBP1-WW for the phosphorylated and active form of the RNAPII-CTD, leading to its dephosphorylation (Okazawa et al., 2002). Desphosphorylation on S2 and diffident reappearance of S5 phosphorylation on the CTD of eleongating RNAPII is known to slow down elongation rates and favor the recognition of weaker consensus sequences for splicing factor (de la Mata et al., 2003). Such situation favors the usage of alternative splicing patterns and the outcome of splicing variated gene products.
Thus, the empirical support for the involvement of PQBP1 in X-LID via alternative RNA splicing is found to fit and complement diverse empirical findings and theoretical postulates independently reported by different research laboratories.
Education
May 2010 – April 2011 |
Fellow scientific associate in “Penelope” (EU Marie Curie Research Training Network, MRTN-CT-2006- 0036076) coordinated by Dr. Luis Serrano, Centre for Genomic Regulation, Barcelona
July 2008 – April 2010 |
Fellow scientific associate in “Structure and Function of Membrane Receptors” (Collaborative Research Centre, SFB 449)
coordinated by Prof. Dr. Volker Haucke, Freie Universität, Berlin
Sept. 2003 – June 2004 |
Diploma thesis in the Systems Immunology Lab
led by Dr. M. Or-Guil at the Institute for theoretical Biology of the Humboldt Universität in collaboration with the Charité Universitätsklinikum, Berlin
Oct. 1998 – June 2004 |
Study of Biology with a Biochemistry major at the Humboldt Universität, Berlin
May 1996 – Sept. 1998 |
German as Foreign Language, German Literature and Philosophy, Epistemology, and Sociobiology as guest student at the RWTH, Aachen.
March 1979 – Dec. 1991 |
School in Viña del Mar, Chile and Philadelphia, PA, USA.
Publications

- 10 - M Spiess, E Verschueren, VE TAPIA, PM Kim, A Norgaard, C Landgraf, R Volkmer, F Hochstenbach, B Distel, B Winsor, L Serrano (2012). “EVOLUTION OF THE SH3 DOMAIN INTERACTOME ACROSS YEAST SPECIES”
- [Manuscript in preparation].
- 9 - Anna Ulbricht, Felix J. Eppler, VE TAPIA, Peter F.M. van der Ven, Padmanabhan Vakeel, Daniela Stadel, Albert Haas, Bernd Hoffmann, Paul Saftig, Christian Behrends, Dieter O. Fürst, Rudolf Volkmer, Waldemar Kolanus & Jörg Höhfeld. (2012) “CELLULAR MECHANOTRANSDUCTION RELIES ON TENSION-INDUCED AND CHAPERONE-ASSISTED AUTOPHAGY“
- [Manuscript submitted on June 29 2012]
- 8 - Volkmer, R & V Tapia. (2011) “EXPLORING PROTEIN-PROTEIN INTERACTIONS WITH SYNTHETIC PEPTIDE ARRAYS”. Mini-Reviews in Organic Chemistry 8(2): 164.
- 7 - Volkmer, R, I Kretzschmar & VE TAPIA. (2011) “Mapping receptor-ligand in-teractions with synthetic peptide arrays: Exploring the structure and function of membrane receptors”. European Journal of Cell Biology 91(4): 349.
- 6 - Tapia VE, Nicolaescu E, McDonald CB, Musi V, Oka T, Inayoshi Y, Satteson AC, Mazack V, Humbert J, Gaffney CJ, Beullens M, Schwartz CE, Landgraf C, Volkmer R, Pastore A, Farooq A, Bollen M, Sudol M. (2010) "Y65C MISSENSE MUTATION IN THE WW DOMAIN OF THE GOLABI-ITO-HALL SYNDROME PROTEIN PQBP1 AFFECTS ITS BINDING ACTIVITY AND DEREGULATES PRE-mRNA SPLICING." J Biol Chem.;285(25):19391-401. Epub 2010 Apr 21.
- This work explores the effect of a point mutation leading to X-LMR at the reductionist level of domain-peptide interactions, inferes potential affected functions, and tests inferences on pre-mRNA splicing.
- Download from JBC
- 5 - Mahrenholz CC, Tapia V, Stigler RD, Volkmer R. (2010) "A STUDY TO ASSESS THE CROSS-REACTIVITY OF CELLULOSE MEMBRANE-BOUND PEPTIDES WITH DETECTION SYSTEMS: AN ANALYSIS AT THE AMINO ACID LEVEL." J Pept Sci.;16(6):297-302.
- ever wanted to see if your favorite detection system reacts with cellulose-bound peptides? Well, chances are given that it is one of the three covered here: TAMRA-dye, luminol turn-over, and FITC
- 4 - Tapia VE & Volkmer R (2009) "EXPLORING AND PROFILING PROTEIN FUNCTION WITH PEPTIDE ARRAYS" in: Methods in Molecular Biology, Vol 570 (Eds. M Cretich & M Chiari). Humana Press. - ISBN: 978-1-60327-393-0.
- This work reviews the peptide array technology focusing on technological advancements, oriented immobilization of peptide probes, and some interesting applications. Going along the philosophy of the monography series, we give some general recomendations on methods and instruments. See the following newsletter comments by Tony Fong: Q&A: Raising the Profile of Peptide Arrays for Studying Protein Function, ProteoMonitor, August 13, 2009.
- View abstract at PubMed
- Read the newsletter by Tony Fong
- 3 - Tapia VE, Ay B, Triebus J, Wolter E, Boisguerin P, Volkmer R. EVALUATING THE COUPLING EFFICIENCY OF PHOSPHORYLATED AMINO ACIDS FOR SPOT SYNTHESIS. J Pept Sci. 2008 Dez ;14(12):1309-1314. - PMID: 18816512.
- This work was carried out to adapt "building block" approaches for the generation of phosphopeptides through resine-supported solid-phase peptide synthesis to the spot-technology, which relies on cellulose as support. It compares different coupling strategies for the incorporation of protected phoshoaminoacids into determined positions of synthetic peptides. We find that the use of EEDQ as coupling activator is the most efficient strategy in our comparison.
- view abstract at PubMed
- 2 - Tapia VE, Bongartz J, Schutkowski M, Bruni N, Weiser A, Ay B, Volkmer R, Or-Guil M. AFFINITY PROFILING USING THE PEPTIDE MICROARRAY TECHNOLOGY: A CASE STUDY. Anal Biochem. 2007 Apr 1;363(1):108-118. - PMID: 17288979.
- This work analyses the predictive potential of microarray-based binding assays. Fluorescent SI-measurements were used to fit a mass-action derived model estimating the observed affinity (equil. diss. const.).
- View abstract at PubMed
- 1 - Weiser AA, Or-Guil M, Tapia VE, Leichsenring A, Schuchhardt J, Frommel C, Volkmer-Engert R. SPOT SYNTHESIS: RELIABILITY OF ARRAY-BASED MEASUREMENT OF PEPTIDE BINDING AFFINITY. Anal Biochem. 2005 Juli 15;342(2):300-311. - PMID: 15950918.
- This work analyses the quantitative potential of measurements derived from peptide macroarray-based immunobinding assays. Chemoluminiscent signal intensity data was confronted with independent "golden stardard" measurements (surface plasmon resonance-based binding assays on a BIAcoreX maschine).